Abstract
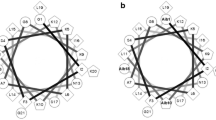
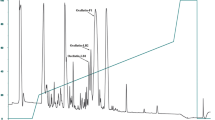
Granular glands in the skins of frogs synthesize and secrete a remarkably diverse range of peptides capable of antimicrobial activity. These anuran skin antimicrobial peptides are commonly hydrophobic, cationic and form an amphipathic α-helix in a membrane mimetic solution. Recently, they have been considered as useful target molecules for developing new antibiotics drugs. Esculentin-1c is a 46-amino acid residue peptide isolated from skin secretions of the European frog, Rana esculenta. It displays the most potent antimicrobial activity among bioactive molecules. Esculentin-1c has the longest amino acids among all antimicrobial peptides. The present study solved the solution structure of esculentin-1c in TFE/water by NMR, for the first time. We conclude that this peptide is comprised of three α-helices with each helix showing amphipathic characteristics, which seems to be a key part for permeating into bacterial membranes, thus presenting antimicrobial activity.
Similar content being viewed by others
References
Ali, M.F., Lips, K.R., Knoop, F.C., Fritzsch, B., Miller, C., and Conlon, J.M. (2002). Antimicrobial peptides and protease inhibitors in the skin secretions of the crawfish frog, Rana areolata. Biochim. Biophys. Acta 1601, 55–63.
Amblard, M., Fehrentz, J.A., Martinez, J., and Subra, G. (2006). Methods and protocols of modern solid phase Peptide synthesis. Mol. Biotechnol. 33, 239–254.
Barra, D., and Simmaco, M. (1995). Amphibian skin: a promising resource for antimicrobial peptides. Trends Biotechnol. 13, 205–209.
Basir, Y.J., Knoop, F.C., Dulka, J., and Conlon, J.M. (2000). Multiple antimicrobial peptides and peptides related to brady-kinin and neuromedin N isolated from skin secretions of the pickerel frog, Rana palustris. Biochim. Biophys. Acta 1543, 95–105.
Bechinger, B. (1997). Structure and functions of channel-forming peptides: Magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 156, 197–211.
Boman, H.G. (1995). Peptide antibiotics and their role in innate immunity. Ann. Rev. Immunol. 13, 61–92.
Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. (1998). Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J., and Bax, A. (1995). NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293.
Dennison, S.R., Wallace, J., Harris, F., and Phoenix, D.A. (2005). Amphiphilic alpha-helical antimicrobial peptides and their structure/function relationships. Protein and Pep. Lett. 12, 31–39.
Gabay, J.E. (1994). Ubiquitous natural antibiotics. Science 264, 373–374.
Gesell, J., Zasloff, M., and Opella, S.J. (1997). Two-dimensional 1H NMR experiments show that the 23-residue magainin antibiotic peptide is an alpha-helix in dodecylphosphocholine micelles, sodium dodecylsulfate micelles, and trifluoroethanol/water solution. J. Biomol. NMR 9, 127–135.
Goraya, J., Knoop, F.C., and Conlon, J.M. (1999). Ranatuerin 1T: an antimicrobial peptide isolated from the skin of the frog, Rana temporaria. Peptides 20, 159–163.
Hancock, R.E. (1997). Peptide antibiotics. Lancet 349, 418–422.
Hancock, R.E.W., and Scott, M.G. (2000). The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97, 8856–8861.
Jenssen, H., Hamill, P., and Hancock, R.E. (2006). Peptide antimicrobial agents. Clin. Microbiol. Rev. 19, 491–511.
Johnson, B.A., and Blevins, R.A. (1994). Nmr view — a computer-program for the visualization and analysis of Nmr data. J. Biomolecular Nmr. 4, 603–614.
Koczulla, A.R., and Bals, R. (2003). Antimicrobial peptides: current status and therapeutic potential. Drugs 63, 389–406.
Mangoni, M.L., Fiocco, D., Mignogna, G., Barra, D., and Simmaco, M. (2003). Functional characterisation of the 1–18 fragment of esculentin-1b, an antimicrobial peptide from Rana esculenta. Peptides 24, 1771–1777.
Mor, A., and Nicolas, P. (1994). The NH2-terminal alpha-helical domain 1–18 of dermaseptin is responsible for antimicrobial activity. J. Biol. Chem. 269, 1934–1939.
Morikawa, N., Hagiwara, K., and Nakajima, T. (1992). Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem. Biophys. Res. Commun. 189, 184–190.
Nascimento, A.C., Fontes, W., Sebben, A., and Castro, M.S. (2003). Antimicrobial peptides from anurans skin secretions. Protein Pept. Lett. 10, 227–238.
Nicolas, P., and Mor, A. (1995). Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 49, 277–304.
Ponti, D., Mignogna, G., Mangoni, M.L., De Biase, D., Simmaco, M., and Barra, D. (1999). Expression and activity of cyclic and linear analogues of esculentin-1, an anti-microbial peptide from amphibian skin. Eur. J. Biochem. 263, 921–927.
Reed, J., and Kinzel, V. (1993). Primary structure elements responsible for the conformational switch in the envelope glycoprotein gp120 from human immunodeficiency virus type 1: LPCR is a motif governing folding. Proc. Natl. Acad. Sci. USA 90, 6761–6765.
Rinaldi, A.C. (2002). Antimicrobial peptides from amphibian skin: an expanding scenario — Commentary. Curr. Opin. Chem. Biol. 6, 799–804.
Rozek, T., Wegener, K.L., Bowie, J.H., Olver, I.N., Carver, J.A., Wallace, J.C., and Tyler, M.J. (2000). The antibiotic and anticancer active aurein peptides from the Australian Bell Frogs Litoria aurea and Litoria raniformis — the solution structure of aurein 1.2. Eur. J. Biochem. 267, 5330–5341.
Shai, Y. (2002). Mode of action of membrane active antimicrobial peptides. Biopolymers 66, 236–248.
Simmaco, M., Mignogna, G., Barra, D., and Bossa, F. (1993). Novel antimicrobial peptides from skin secretion of the European frog Rana esculenta. FEBS Lett. 324, 159–161.
Simmaco, M., Mignogna, G., Barra, D., and Bossa, F. (1994). Antimicrobial peptides from skin secretions of Rana esculenta. Molecular cloning of cDNAs encoding esculentin and brevinins and isolation of new active peptides. J. Biol. Chem. 269, 11956–11961.
Simmaco, M., Mignogna, G., and Barra, D. (1998). Antimicrobial peptides from amphibian skin: what do they tell us. Biopolymers 47, 435–450.
Sivaraman, T., Kumar, T.K., and Yu, C. (1996). Destabilisation of native tertiary structural interactions is linked to helix-induction by 2,2,2-trifluoroethanol in proteins. Int. J. Biol. Macromol. 19, 235–239.
Sonnichsen, F.D., Van Eyk, J.E., Hodges, R.S., and Sykes, B.D. (1992). Effect of trifluoroethanol on protein secondary structure: an NMR and CD study using a synthetic actin peptide. Biochemistry 31, 8790–8798.
Vanhoye, D., Bruston, F., Nicolas, P., and Amiche, M. (2003). Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur. J. Biochem. 270, 2068–2081.
Vignal, E., Chavanieu, A., Roch, P., Chiche, L., Grassy, G., Calas, B., and Aumelas, A. (1998). Solution structure of the antimicrobial peptide ranalexin and a study of its interaction with perdeuterated dodecylphosphocholine micelles. Eur. J. Biochem. 253, 221–228.
Wellings, D.A., and Atherton, E. (1997). Standard Fmoc protocols. Methods Enzymol. 289, 44–67.
Wishart, D.S., and Sykes, B.D. (1994a). The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR 4, 171–180.
Wishart, D.S., and Sykes, B.D. (1994b). Chemical shifts as a tool for structure determination. Methods Enzymol. 239, 363–392.
Wishart, D.S., Bigam, C.G., Yao, J., Abildgaard, F., Dyson, H.J., Oldfield, E., Markley, J.L., and Sykes, B.D. (1995). 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR 6, 135–140.
Won, H.S., Park, S.H., Kim, H.E., Hyun, B., Kim, M., and Lee, B.J. (2002). Effects of a tryptophanyl substitution on the structure and antimicrobial activity of C-terminally truncated gaegurin 4. Eur. J. Biochem. 269, 4367–4374.
Won, H.S., Jung, S.J., Kim, H.E., Seo, M.D., and Lee, B.J. (2004a). Systematic peptide engineering and structural characterization to search for the shortest antimicrobial peptide analogue of gaegurin 5. J. Biol. Chem. 279, 14784–14791.
Won, H.S., Kim, S.S., Jung, S.J., Son, W.S., Lee, B., and Lee, B.J. (2004b). Structure activity relationships of antimicrobial peptides from the skin of Rana esculenta inhabiting in Korea. Mol. Cells 17, 469–476.
Won, H.S., Kang, S.J., and Lee, B.J. (2009). Action mechanism and structural requirements of the antimicrobial peptides, gaegurins. Biochim. Biophys. Acta 1788, 1620–1629.
Wüthrich, K. (1986). NMR of proteins and nucleic acids (New York, USA: John Wiley & Sons).
Zasloff, M. (1987). Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 84, 5449–5453.
Zasloff, M. (2002). Antimicrobial peptides of multicellular organisms. Nature 415, 389–395.
Zasloff, M. (2003). Vernix, the newborn, and innate defense. Pediatr. Res. 53, 203–204.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kang, SJ., Son, WS., Han, KD. et al. Solution structure of antimicrobial peptide esculentin-1c from skin secretion of Rana esculenta . Mol Cells 30, 435–441 (2010). https://doi.org/10.1007/s10059-010-0135-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-010-0135-7