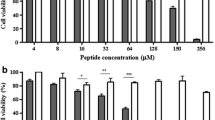
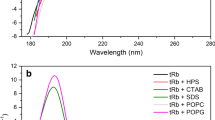
Abstract
Antimicrobial peptides (AMPs) play a key role in the defence mechanism of living organisms against microbial pathogens, displaying both bactericidal and immunomodulatory properties. They are considered as a promising alternative to the conventional antibiotics towards which bacteria are becoming highly resistant. Recently, a derivative of the frog skin AMP esculentin-1a, esculentin-1a(1–21)NH2 [Esc(1–21)], showed a strong and fast membranolytic activity against Gram-negative bacteria but with a lower efficacy against Gram-positive ones. Here, with the aim to increase the α-helicity of Esc(1–21) and the expected potency against Gram-positive bacteria, we designed an analog bearing three α-aminoisobutyric acid (Aib) residues at positions 1, 10, and 18 of its primary structure. We demonstrated that the incorporation of Aib residues: (1) promoted the α-helix conformation of Esc(1–21), as confirmed by circular dichroism and two-dimensional nuclear magnetic resonance spectroscopies; (2) was sufficient to make this analog more active than the parent peptide against several Gram-positive bacterial strains without affecting its activity against Gram-negative bacteria; and (3) resulted to be devoid of toxic effect toward epithelial cells at the active antimicrobial concentrations. These results suggest that replacement of L-amino acids with Aib residues has beneficial effects on the structure and properties of the membrane-active peptide Esc(1–21), making it a better candidate for the design and development of selective drugs against Gram-positive bacteria.





Similar content being viewed by others
Abbreviations
- CD:
-
Circular dichroism
- DIEA:
-
N,N-Diisopropylethylamine
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Heat-inactivated fetal bovine serum
- HATU:
-
1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate
- HBTU:
-
N,N,N′,N′-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate
- HOBt:
-
1-Hydroxybenzotriazole
- MBHA:
-
4-Methylbenzhydrylamine
- MH:
-
Mueller–Hinton
- MTT:
-
3(4,5-Dimethylthiazol-2yl)2,5-diphenyltetrazolium bromide
- NMR:
-
Nuclear magnetic resonance
- SDS:
-
Sodium dodecylsulfate
- TIS:
-
Triisopropylsilane
- TFA:
-
Trifluoroacetic acid
- TFE:
-
Trifluoroethanol
References
Abbouda A, Abicca I, Alio JL (2016) Infectious keratitis following corneal crosslinking: a systematic review of reported cases: management, visual outcome, and treatment proposed. Semin Ophthalmol 31:485–491
Bai H, Zhou Y, Hou Z, Xue X, Meng J, Luo X (2011) Targeting bacterial RNA polymerase: promises for future antisense antibiotics development. Infect Disord Drug Targets 11:175–187
Bax A, Davis DG (1985) MLEV-17-based two dimensional homonuclear magnetization transfer spectroscopy. J Magn Reson 65:355–360
Beychock S (1967) Circular dichroism of poly-α-amino acids and proteins. In: Fasman GD (ed) Poly-α-amino acids: protein models for conformational studies. Dekker, New York, pp 293–337
Bhunia A, Ramamoorthy A, Bhattacharjya S (2009) Helical hairpin structure of a potent antimicrobial peptide MSI-594 in lipopolysaccharide micelles by NMR spectroscopy. Chemistry 15:2036–2040
Bhunia A, Domadia PN, Torres J, Hallock KJ, Ramamoorthy A, Bhattacharjya S (2010) NMR structure of pardaxin, a pore-forming antimicrobial peptide, in lipopolysaccharide micelles: mechanism of outer membrane permeabilization. J Biol Chem 285:3883–3895
Bodey GP, Bolivar R, Fainstein V, Jadeja L (1983) Infections caused by Pseudomonas aeruginosa. Rev Infect Dis 5:279–313
Breidenstein EB, de la Fuente-Nunez C, Hancock RE (2011) Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol 19:419–426
Chen T, Farragher S, Bjourson AJ, Orr DF, Rao P, Shaw C (2003) Granular gland transcriptomes in stimulated amphibian skin secretions. Biochem J 371:125–130
Choi KY, Chow LN, Mookherjee N (2012) Cationic host defence peptides: multifaceted role in immune modulation and inflammation. J Innate Immun 4:361–370
Conlon JM (2011) Structural diversity and species distribution of host-defense peptides in frog skin secretions. Cell Mol Life Sci 68:2303–2315
Dathe M, Wieprecht T, Nikolenko H, Handel L, Maloy WL, MacDonald DL, Beyermann M, Bienert M (1997) Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett 403:208–212
Dathe M, Schumann M, Wieprecht T, Winkler A, Beyermann M, Krause E, Matsuzaki K, Murase O, Bienert M (1996) Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry 35:12612–12622
De Zotti M, Biondi B, Formaggio F, Toniolo C, Stella L, Park Y, Hahm KS (2009) Trichogin GA IV: an antibacterial and protease-resistant peptide. J Pept Sci 15:615–619
De Zotti M, Biondi B, Park Y, Hahm KS, Crisma M, Toniolo C, Formaggio F (2012) Antimicrobial lipopeptaibol trichogin GA IV: role of the three Aib residues on conformation and bioactivity. Amino Acids 43:1761–1777
Di Grazia A, Cappiello F, Imanishi A, Mastrofrancesco A, Picardo M, Paus R, Mangoni ML (2015a) The frog skin-derived antimicrobial peptide esculentin-1a(1–21)NH2 promotes the migration of human HaCaT keratinocytes in an EGF receptor-dependent manner: a novel promoter of human skin wound healing? PLoS One 10:e0128663
Di Grazia A, Cappiello F, Cohen H, Casciaro B, Luca V, Pini A, Di YP, Shai Y, Mangoni ML (2015b) D-Amino acids incorporation in the frog skin-derived peptide esculentin-1a(1–21)NH2 is beneficial for its multiple functions. Amino Acids 47:2505–2519
Domadia PN, Bhunia A, Ramamoorthy A, Bhattacharjya S (2010) Structure, interactions, and antibacterial activities of MSI-594 derived mutant peptide MSI-594F5A in lipopolysaccharide micelles: role of the helical hairpin conformation in outer-membrane permeabilization. J Am Chem Soc 132:18417–18428
Drenkard E, Ausubel FM (2002) Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740–743
Epand RF, Savage PB, Epand RM (2007) Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins). Biochim Biophys Acta 1768:2500–2509
Epand RM, Vogel HJ (1999) Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta 1462:11–28
Erspamer V (1971) Biogenic amines and active polypeptides of the amphibian skin. Annu Rev Pharmacol 11:327–350
Free SJ (2013) Fungal cell wall organization and biosynthesis. Adv Genet 81:33–82
Gamberi T, Cavalieri D, Magherini F, Mangoni ML, De Filippo C, Borro M, Gentile G, Simmaco M, Modesti A (2007) An integrated analysis of the effects of Esculentin 1–21 on Saccharomyces cerevisiae. Biochim Biophys Acta 1774:688–700
Ganz T, Lehrer RI (1998) Antimicrobial peptides of vertebrates. Curr Opin Immunol 10:41–44
Gazit E, Lee WJ, Brey PT, Shai Y (1994) Mode of action of the antibacterial cecropin B2: a spectrofluorometric study. Biochemistry 33:10681–10692
Ghosh A, Bera S, Shai Y, Mangoni ML, Bhunia A (2016) NMR structure and binding of esculentin-1a (1–21)NH2 and its diastereomer to lipopolysaccharide: correlation with biological functions. Biochim Biophys Acta 1858:800–812
Giangaspero A, Sandri L, Tossi A (2001) Amphipathic alpha helical antimicrobial peptides. Eur J Biochem 268:5589–5600
Glukhov E, Stark M, Burrows LL, Deber CM (2005) Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J Biol Chem 280:33960–33967
Godwin U, Akpan MGS (2014) Effects of different concentrations of biocides on biofilm microorganisms in oil pipelines in Irri, Delta State, Nigeria. Int J Biosci 4:16
Gonzalez-Navajas JM, Corr MP, Raz E (2014) The immediate protective response to microbial challenge. Eur J Immunol 44:2536–2549
Griesinger COG, Wüthrich K, Ernst RR (1988) Clean TOCSY for proton spin system identification in macromolecules. J Am Chem Soc 110:7870–7872
Hancock RE, Rozek A (2002) Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol Lett 206:143–149
Hancock RE, Haney EF, Gill EE (2016) The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol 16:321–334
Haney EF, Nathoo S, Vogel HJ, Prenner EJ (2010) Induction of non-lamellar lipid phases by antimicrobial peptides: a potential link to mode of action. Chem Phys Lipids 163:82–93
Haslam IS, Roubos EW, Mangoni ML, Yoshizato K, Vaudry H, Kloepper JE, Pattwell DM, Maderson PF, Paus R (2014) From frog integument to human skin: dermatological perspectives from frog skin biology. Biol Rev Camb Philos Soc 89:618–655
Hemshekhar M, Anaparti V, Mookherjee N (2016) Functions of cationic host defense peptides in immunity. Pharmaceuticals 9(3)
Hoiby N, Ciofu O, Johansen HK, Song ZJ, Moser C, Jensen PO, Molin S, Givskov M, Tolker-Nielsen T, Bjarnsholt T (2011) The clinical impact of bacterial biofilms. Int J Oral Sci 3:55–65
Islas-Rodriguez AE, Marcellini L, Orioni B, Barra D, Stella L, Mangoni ML (2009) Esculentin 1–21: a linear antimicrobial peptide from frog skin with inhibitory effect on bovine mastitis-causing bacteria. J Pept Sci 15:607–614
Karle IL, Balaram P (1990) Structural characteristics of alpha-helical peptide molecules containing Aib residues. Biochemistry 29:6747–6756
Kolar SS, Luca V, Baidouri H, Mannino G, McDermott AM, Mangoni ML (2015) Esculentin-1a(1–21)NH: a frog skin-derived peptide for microbial keratitis. Cell Mol Life Sci 72:617–627
Konig E, Bininda-Emonds OR, Shaw C (2014) The diversity and evolution of anuran skin peptides. Peptides 63C:96–117
Lee YR, Houngue C, Hall RG (2015) Treatment of community-acquired pneumonia. Expert Rev Anti Infect Ther 13:1109–1121
Levy SB (2002) The 2000 Garrod lecture. Factors impacting on the problem of antibiotic resistance. J Antimicrob Chemother 49:25–30
Lohner K (2016) Novel antibiotics based upon the multiple mechanisms of membrane perturbation by antimicrobial peptides. Curr Top Med Chem (in press)
Lohner K, Blondelle SE (2005) Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb Chem High Throughput Screen 8:241–256
Luca V, Stringaro A, Colone M, Pini A, Mangoni ML (2013) Esculentin(1–21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cell Mol Life Sci 70:2773–2786
Mangoni ML (2006) Temporins, anti-infective peptides with expanding properties. Cell Mol Life Sci 63:1060–1069
Mangoni ML, Shai Y (2011) Short native antimicrobial peptides and engineered ultrashort lipopeptides: similarities and differences in cell specificities and modes of action. Cell Mol Life Sci 68:2267–2280
Mangoni ML, Marcellini HG, Simmaco M (2007) Biological characterization and modes of action of temporins and bombinins H, multiple forms of short and mildly cationic anti-microbial peptides from amphibian skin. J Pept Sci 13:603–613
Mangoni ML, Luca V, McDermott AM (2015) Fighting microbial infections: A lesson from amphibian skin-derived esculentin-1 peptides. Peptides 71:286–295
Mangoni ML, Miele R, Renda TG, Barra D, Simmaco M (2001) The synthesis of antimicrobial peptides in the skin of Rana esculenta is stimulated by microorganisms. FASEB J 15:1431–1432
Mangoni ML, Fiocco D, Mignogna G, Barra D, Simmaco M (2003) Functional characterisation of the 1-18 fragment of esculentin-1b, an antimicrobial peptide from Rana esculenta. Peptides 24:1771–1777
Manning MC, Woody RW (1991) Theoretical CD studies of polypeptide helices: examination of important electronic and geometric factors. Biopolymers 31:569–586
Mansour SC, Pena OM, Hancock RE (2014) Host defense peptides: front-line immunomodulators. Trends Immunol 35:443–450
Matsuzaki K (2009) Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta 1788:1687–1692
Mookherjee N, Hancock RE (2007) Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci 64:922–933
Nicolas P, Mor A (1995) Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu Rev Microbiol 49:277–304
Paiva AD, de Oliveira MD, de Paula SO, Baracat-Pereira MC, Breukink E, Mantovani HC (2012) Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology 158:2851–2858
Parsek MR, Tolker-Nielsen T (2008) Pattern formation in Pseudomonas aeruginosa biofilms. Curr Opin Microbiol 11:560–566
Ponti D, Mangoni ML, Mignogna G, Simmaco M, Barra D (2003) An amphibian antimicrobial peptide variant expressed in Nicotiana tabacum confers resistance to phytopathogens. Biochem J 370:121–127
Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y (1992) Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry 31:12416–12423
Rance M, Sorensen OW, Bodenhausen G, Wagner G, Ernst RR, Wuthrich K (1983) Improved spectral resolution in cosy 1H NMR spectra of proteins via double quantum filtering. Biochem Biophys Res Commun 117:479–485
Rink R, Arkema-Meter A, Baudoin I, Post E, Kuipers A, Nelemans SA, Akanbi MH, Moll GN (2010) To protect peptide pharmaceuticals against peptidases. J Pharmacol Toxicol Methods 61:210–218
Rybtke M, Hultqvist LD, Givskov M, Tolker-Nielsen T (2015) Pseudomonas aeruginosa Biofilm Infections: Community Structure, Antimicrobial Tolerance and Immune Response. J Mol Biol 427:3628–3645
Savjani JK, Gajjar AK, Savjani KT (2009) Mechanisms of resistance: useful tool to design antibacterial agents for drug—resistant bacteria. Mini Rev Med Chem 9:194–205
Schaffer C, Messner P (2005) The structure of secondary cell wall polymers: how Gram-positive bacteria stick their cell walls together. Microbiology 151:643–651
Schwermer CU, Lavik G, Abed RM, Dunsmore B, Ferdelman TG, Stoodley P, Gieseke A, de Beer D (2008) Impact of nitrate on the structure and function of bacterial biofilm communities in pipelines used for injection of seawater into oil fields. Appl Environ Microbiol 74:2841–2851
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolymers 66:236–248
Shai Y, Oren Z (1996) Diastereoisomers of cytolysins, a novel class of potent antibacterial peptides. J Biol Chem 271:7305–7308
Simmaco M, Mignogna G, Barra D, Bossa F (1994) Antimicrobial peptides from skin secretions of Rana esculenta. Molecular cloning of cDNAs encoding esculentin and brevinins and isolation of new active peptides. J Biol Chem 269:11956–11961
Soufi Y, Soufi B (2016) Mass spectrometry-based bacterial proteomics: focus on dermatologic microbial pathogens. Front Microbiol 7:181
Strahilevitz J, Mor A, Nicolas P, Shai Y (1994) Spectrum of antimicrobial activity and assembly of dermaseptin-b and its precursor form in phospholipid membranes. Biochemistry 33:10951–10960
Toniolo C, Crisma M, Formaggio F, Peggion C (2001) Control of peptide conformation by the Thorpe-Ingold effect (C alpha-tetrasubstitution). Biopolymers 60:396–419
Toniolo C, Peggion C, Crisma M, Formaggio F, Shui X, Eggleston DS (1994) Structure determination of racemic trichogin A IV using centrosymmetric crystals. Nat Struct Biol 1:908–914
Toniolo C, Brückner H (2009) Peptaibiotics: fungal peptides containing α-dialkyl α-amino Acids. Wiley-VCH and VHCA, Weinheim/Zürich. Chem Biochem 10:2266–2267
Uccelletti D, Zanni E, Marcellini L, Palleschi C, Barra D, Mangoni ML (2010) Anti-Pseudomonas activity of frog skin antimicrobial peptides in a Caenorhabditis elegans infection model: a plausible mode of action in vitro and in vivo. Antimicrob Agents Chemother 54:3853–3860
Valenti P, Visca P, Antonini G, Orsi N (1985) Antifungal activity of ovotransferrin towards genus Candida. Mycopathologia 89:169–175
Veber DF, Freidinger RM (1985) The design of metabolically-stable peptide analogs. Trends Neurosci 8:392–396
Wüthrich K (1986) NMR of proteins and nucleic acids. Wiley, New York
Yamaguchi H, Kodama H, Osada S, Kato F, Jelokhani-Niaraki M, Kondo M (2003) Effect of alpha, alpha-dialkyl amino acids on the protease resistance of peptides. Biosci Biotechnol Biochem 67:2269–2272
Acknowledgments
This work was supported by grants from Sapienza Università di Roma and by FILAS Grant Prot. FILAS-RU-2014-1020. This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: M. S. Palma.
Rights and permissions
About this article
Cite this article
Biondi, B., Casciaro, B., Di Grazia, A. et al. Effects of Aib residues insertion on the structural–functional properties of the frog skin-derived peptide esculentin-1a(1–21)NH2 . Amino Acids 49, 139–150 (2017). https://doi.org/10.1007/s00726-016-2341-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2341-x