Abstract
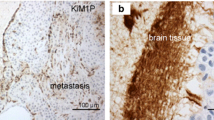
The process of metastasis consists of a series of sequential, selective steps that few cells can complete. The outcome of cancer metastasis depends on multiple interactions between metastatic cells and homeostatic mechanisms that are unique to one or another organ microenvironment. The specific organ microenvironment determines the extent of cancer cell proliferation, angiogenesis, invasion and survival. Many lung cancer, breast cancer, and melanoma patients develop fatal brain metastases that do not respond to therapy. The blood-brain barrier is intact in and around brain metastases that are smaller than 0.25 mm in diameter. Although the blood-brain barrier is leaky in larger metastases, the lesions are resistant to many chemotherapeutic drugs. Activated astrocytes surround and infiltrate brain metastases. The physiological role of astrocytes is to protect against neurotoxicity. Our current data demonstrate that activated astrocytes also protect tumor cells against chemotherapeutic drugs.
Similar content being viewed by others
References
Abbott, N.J., Rönnbäck, L., and Hansson, E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53.
Allen, N.J., and Barres, B.A. (2009). Neuroscience: glia - more than just brain glue. Nature 457, 675–677.
Aukerman, S.L., Price, J.E., and Fidler, I.J. (1986). Different deficiencies in the prevention of tumorigenic-low-metastatic murine K-1735 melanoma cells from producing metastasis. J. Natl. Cancer Inst. 77, 915–924.
Brown, J.M., and Giaccia, A.J. (1998). The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 58, 1408–1416.
Bullock, T.H., Bennett, M.V., Johnston, D., Josephson, R., Marder, E., and Fields, R.D. (2005). Neuroscience. The neuron doctrine, redux. Science 310, 791–793.
Carmeliet, P., Ferreira, V., Breier, G., Pollefeyt, S., Kieckens, L., Gerstenstein, M., Fahrig, M., Vandenhoeck, A., Harpal, K., Eberhardt, C., et al. (1996). Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439.
Carmeliet, P., and Jain, R.K. (2000). Angiogenesis in cancer and other diseases. Nature 407, 249–257.
Chalkley, H.W. (1943). Method for the quantitative morphologic analysis of tissues. J. Natl. Cancer Inst. 4, 47–53.
Chen, L.W., Yung, K.L., and Chan, Y.S. (2005). Reactive astrocytes as potential manipulation targets in novel cell replacement therapy of Parkinson’s disease. Curr. Drug Targets 6, 821–833.
Crooks, D.A., Scholtz, C.L., Vowles, G., Greenwald, S., and Evans, S. (1991). The glial reaction in closed head injuries. Neuropathol. Appl. Neurobiol. 17, 407–414.
Ewing, J. (1928). Neoplastic Diseases, 6th eds., W. B. Saunders, (Philadelphia, USA).
Feigin, I., Allen, L.B., Lipkin, L., and Gross, S.W. (1958). The endothelial hyperplasia of the cerebral blood vessels with brain tumors and its sarcomatous transformation. Cancer 11, 264–277.
Felgenhauer, K. (1986). The blood-brain barrier redefined. J. Neurol. 233, 193–194.
Ferrara, N., and Henzel, W.J. (1989). Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 161, 851–859.
Fidler, I.J. (1973). Selection of successive tumour lines for metastasis. Nat. New Biol. 242, 148–149.
Fidler, I.J. (1990). Critical factors in the biology of human cancer metastasis: twenty-eighth GHA Clowes memorial award lecture. Cancer Res. 50, 6130–6138.
Fidler, I.J. (2003). The pathogenesis of cancer metastasis: the ’seed and soil’ revisited (Timeline). Nat. Rev. Cancer 3, 453–458.
Fidler, I.J., and Kripke, M.E. (1977). Metastasis results from pre-existing variant cells within a malignant tumor. Science 197, 893–895.
Fidler, I.J., and Talmadge, J.E. (1986). Evidence that intravenously derived murine pulmonary melanoma metastases can originate from the expansion of a single tumor cell. Cancer Res. 46, 5167–5171.
Fidler, I.J., Yano, S., Zhang, R.D., Fujimaki, T., and Bucana, C.D. (2002). The seed and soil hypothesis: vascularization and brain metastasis. Lancet Oncol. 3, 53–57.
Fields, R.D., and Stevens-Graham, B. (2002). New insights into neuron-glia communication. Science 298, 556–562.
Folkman, J. (1995). Clinical approaches of research on angiogenesis. N. Engl. J. Med. 333, 1757–1763.
Fujimaki, T., Fan, D., Staroselsky, A.H., Gohji, K., Bucana, C.D., and Fidler, I.J. (1993). Critical factors regulating site-specific brain metastasis of murine melanomas. Int. J. Oncol. 3, 789–799.
Gavrieli, Y., Sherman, Y., and Ben-Sasson, S.A. (1992). Identification of programmed cell death in situ via a specific labeling of nuclear DNA fragmentation. J. Cell Biol.119, 493–501.
Gay, P.C., Litchy, W.J., and Carcino, T.L. (1987). Brain metastasis in hypernephroma. J. Neurooncol. 5, 51–56.
Gregoire, N. (1989). The blood-brain barrier. J. Neuroradiol. 16, 238–250.
Greig, N.H., Soncrant, T.T., Shetty, H.U., Momma, S., Smith, Q.R., and Rapoport, S.I. (1990). Brain uptake and anticancer activities of vincristine and vinblastine are restricted by their low cerebrovascular permeability and binding to plasma constituents in rat. Cancer Chemother. Pharmacol. 26, 263–268.
Groothuis, D.R., Fischer, J.M., Lapin, G., Bigner, D.D., and Vick, N.A. (1982). Permeability of different experimental brain tumor models to horseradish peroxidase. J. Neuropathol. Exp. Neurol. 41, 164–185.
Hart, I.R., Talmadge, J.E., and Fidler, I.J. (1981). Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 41, 1281–1287.
Holash, J., Maisonpierre, P.C., Compton, D., Boland, P., Alexander, C.R., Zagzag, D., Yancopoulos, G.D., and Wiegand, S.J. (1999). Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998.
Hu, F., Wang, R.Y., and Hsu, T.C. (1987). Clonal origin of metastasis in B16 murine melanoma: a cytogenetic study. J. Natl. Cancer Inst. 67, 947–956.
Jain, R.K. (2001). Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Del. Rev. 46, 149–168.
Johansson, B.B. (1990). The physiology of the blood-brain barrier. Adv. Exp. Med. Biol. 274, 25–39.
Kanematsu, T., Matsumata, T., Takenaka, K., Yoshida, Y., Higashi, H., and Sugimachi, K. (1988). Clinical management of recurring hepatocellular carcinoma after primary resection. Br. J. Surg. 75, 203–206.
Kim, S.J., Baker, C.H., Kitadia, Y., Nakamuyra, T., Kuwai, T., Sasaki, T., Langley, R., and Fidler, I.J. (2010). Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. In Proc. 101st Ann. Meeting of the Amer. Assoc. Cancer Res., 2010 Apr 17–21, Washington, DC. Abst nr 3428.
Landis, S.H., Murray, T., Bolden, S., and Wingo, P.A. (1998). Cancer statistics. CA Cancer J. Clin. 48, 6–29.
Langley, R.R., and Fidler, I.J. (2007). Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr. Rev. 28, 297–321.
Langley, R.R., Fan, D., Guo, L., Zhang, C., Lin, Q., Brantley, E.C., McCarty, J.H., and Fidler, I.J. (2009). Generation of an immortalized astrocyte cell line from H-2K b-tsA58 mice to study the role of astrocytes in brain metastasis. Int. J. Oncol. 35, 665–672.
Li, L., Price, J.E., Fan., D., Zhang, R., Bucana, C.D., and Fidler, I.J. (1989). Correlation of growth capacity of human tumor cells in hard agarose with their in vivo proliferative capacity at specific metastatic sites. J. Natl. Cancer Inst. 81, 1406–1412.
Lin, Q., Balasubramanian, K.K., Fan, D., Kim, S.J., Guo, L., Wang, H., Bar-Eli, M., Aldape, K.D., and Fidler, I.J. (2010). Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia (in press).
Liotta, L.A., Steeg, P.S., and Stetler-Stevenson, W.G. (1991). Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64, 327–332.
Mahesh, V.B., Dhandapani, K.M., and Brann, D.W. (2006). Role of astrocytes in reproduction and neuroprotection. Mol. Cell. Endocrinol. 246, 1–9.
Miller, G. (2005). Neuroscience: the dark side of glia. Science 308, 778–781.
Nagy, J.A., Morgan, E.S., Herzberg, K.T., Manseau, E.J., Dvorak, A.M., and Dvorak, H.F. (1995). Pathogenesis of ascites tumor growth: angiogenesis, vascular remodeling, and stroma formation in the peritoneal lining. Cancer Res. 55, 376–385.
Nicolson, G. (1988). Organ specificity of tumor metastasis: role of preferential adhesion, invasion, and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev. 7, 143–188.
Norden, A.D., Wen, P.Y., and Kesari, S. (2005). Brain metastasis. Curr. Opin. Neurol. 18, 654–661.
Paget, S. (1889). The distribution of secondary growths in cancer of the breast. Lancet 1, 571–573.
Patan, S. (1998). Tie1 and Tie2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc. Res. 56, 1–21.
Patan, S., Munn, L.L., and Jain, R.K. (1996). Intussusceptive microvascular growth in a human colon adenocarcinoma xenograft: a novel mechanism of tumor angiogenesis. Microvasc. Res. 51, 260–272.
Poste, G., and Fidler, I.J. (1980). The pathogenesis of cancer metastasis. Nature 283, 139–146.
Risau, W. (1997). Mechanisms of angiogenesis. Nature 386, 671–674.
Russ, J.C. (1989). Uses of the Euclidean distance map for the measurement of features in images. J. Comp. Assist. Miscrosc. 1, 343–375.
Russ, J.C. (1995). The Image Processing Handbook, 2nd ed., (Boca Raton, USA: CRC Press), pp. 463–469.
Sawaya, R., Bindal, R., and Lang, F.F. (2001). Metastatic brain tumors. In Brian Tumors, A.H. Kaye, and E.E. Laws, eds. (New York, USA: Churchill-Livingsone), pp. 999–1026.
Schackert, G., and Fidler, I.J. (1988a). Development of in vivo models for studies of brain metastasis. Int. J. Cancer 41, 589–594.
Schackert, G., and Fidler, I.J. (1988b). Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res. 48, 3478–3484
Schackert, G., Simmons, R.D., Buzbee, T.M., Hume, D.A., and Fidler, I.J. (1988). Macrophage infiltration into experimental brain metastasis: occurrence through an intact blood-brain barrier. J. Natl. Cancer Inst. 80, 1027–1034.
Senger, D.R., Galli, S.J., Dvorak, A.M., Perruzzi, C.A., Harvey, V.S., and Dvorak, H.F. (1983). Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985.
Schlingemann, R.O., Rietveld, F.J., de Waal, R.M., Ferrone, S., and Ruiter, D.J. (1990). Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am. J. Pathol. 136, 1393–1405.
Shapiro, W.R., and Shapiro, J.R. (1986). Principles of brain tumor chemotherapy. Semin. Oncol. 1, 56–69.
Sofroniew, M.V. (2005). Reactive astrocytes in neural repair and protection. Neuroscientist 11, 400–407.
Stewart, P.A., Hayakawa, K., Farrell, C.L., and Del Maestro, R.F. (1987). Quantitative study of microvessel ultrastructure in human peritumoral brain tissue: evidence for a blood-brain barrier defect. J. Neurosurg. 67, 697–705.
Talmadge, J.E., and Fidler, I.J. (1982). Cancer metastasis is selective or random depending on the parent tumor population. Nature 27, 593–594.
Talmadge, J.E., and Fidler, I.J. (2010). AACR Centennial Series: The biology of cancer metastasis: historical perspective. Cancer Res. 70, 5649–5669.
Talmadge, J.E., Wolman, S.R., and Fidler, I.J. (1982). Evidence for the clonal origin of spontaneous metastasis. Science 217, 361–363.
Tannock, I.F. (1968). The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br. J. Cancer 22, 258–273.
Tarin, D., Price, J.E., Kettlewell, M.G.W., Souter, R.G., Vass, A.C., and Crossley, B. (1984). Mechanisms of human tumor metastasis studied in patients with peritoneouvenous shunts. Cancer Res. 44, 3584–3591.
Tomlinson, E. (1987). Theory and practice of site-specific drug delivery. Adv. Drug Deliv. Rev. 1, 187–198.
Tsukuda, T., Fouad, A., and Pickren, J.W. (1983). Central nervous system metastasis from breast carcinoma: autopsy study. Cancer 52, 2349–2354.
Unemori, E.N., Ferrara, N., Bauer, E.A., and Amento, E.P. (1992). Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J. Cell. Physiol. 153, 557–562.
Viadana, E., Bross, I.D.J., and Pickren, J.W. (1978). The metastatic spread of cancers of the digestive system in man. Oncology 35, 114–126.
Weidner, N. (1998). Tumoral vascularity as a prognostic factor in cancer patients: the evidence continues to grow (Editorial). Am. J. Pathol. 184, 130–135.
Weiss, L. (1985). Principles of Metastasis (Orlando, USA: Academic Press).
Yano, S., Shinohara, H., Herbst, R.S., Kuniyasu, H., Bucana, C.D., Ellis, L.M., Davis, D.W., McConkey, D.J., and Fidler, I.J. (2000). Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 260, 4959–4967.
Zagzag, D., Goldenberg, M., and Brem, S. (1989). Angiogenesis and blood-brain barrier breakdown modulate CT contrast enhancement: an experimental study in a rabbit brain-tumor model. Am. J. Roentgenol. 153, 141–146.
Zhang, R.D., Price, J.E., Schackert, G., Itoh, K., and Fidler, I.J. (1991). Malignant potential of cells isolated from lymph node or brain metastases of melanoma patients and implications of prognosis. Cancer Res. 51, 2029–2035.
Zhang, R.D., Price, J.E., Fujimaki, T., Bucana, C.D., and Fidler IJ.. (1992). Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am. J. Pathol. 141, 1115–1124.
Zuelch, K.G. (1986). Brain tumors: their biology and pathology, 3rd eds., (Berlin, Germany: Springer-Verlag), pp. 480–498.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fidler, I.J., Balasubramanian, K., Lin, Q. et al. The brain microenvironment and cancer metastasis. Mol Cells 30, 93–98 (2010). https://doi.org/10.1007/s10059-010-0133-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-010-0133-9