Abstract
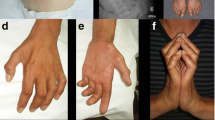
Inherited disorders characterized by motor neuron loss and muscle weakness are genetically heterogeneous. The recent identification of mutations in the gene encoding transient receptor potential vanilloid 4 (TRPV4) in distal spinal muscular atrophy (dSMA) prompted us to screen for TRPV4 mutations in a small group of children with compatible phenotype. In a girl with dSMA and vocal cord paralysis, we detected a new variant (p.P97R) localized in the cytosolic N-terminus of the TRPV4 protein, upstream of the ankyrin-repeat domain, where the great majority of disease-associated mutations reside. In another child with congenital dSMA, in this case associated with bone abnormalities, we detected a previously reported mutation (p.R232C). Functional analysis of the novel p.P97R mutation in a heterologous system demonstrated a loss-of-function mechanism. Protein localization studies in muscle, skin, and cultured skin fibroblasts from both patients showed normal protein expression. No TRPV4 mutations were detected in four children with dSMA without bone or vocal cord involvement. Adding to the clinical and molecular heterogeneity of TRPV4-associated diseases, our results suggest that molecular testing of the TRPV4 gene is warranted in cases of congenital dSMA with bone abnormalities and vocal cord paralysis.





Similar content being viewed by others
References
Wee CD, Kong L, Sumner CJ (2010) The genetics of spinal muscular atrophies. Curr Opin Neurol 23:450–458
Auer-Grumbach M, Olschewski A, Papic L et al (2010) Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet 42:160–164
Landouré G, Zdebik AA, Martinez TL et al (2010) Mutations in TRPV4 cause Charcot–Marie–Tooth disease type 2C. Nat Genet 42:170–174
Deng HX, Klein CJ, Yan J et al (2010) Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet 42:165–169
Zimoń M, Baets J, Auer-Grumbach M et al (2010) Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain 133:1798–1809
Klein CJ, Shi Y, Fecto F et al (2011) TRPV4 mutations and cytotoxic hypercalcemia in axonal Charcot–Marie–Tooth neuropathies. Neurology 76:887–894
Krakow D, Vriens J, Camacho N et al (2009) Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am J Hum Genet 84:307–315
Andreucci E, Aftimos S, Alcausin M et al (2011) TRPV4 related skeletal dysplasias: a phenotypic spectrum highlighted by clinical, radiographic, and molecular studies in 21 new families. Orphanet J Rare Dis 6:37
Camacho N, Krakow D, Johnykutty S et al (2010) Dominant TRPV4 mutations in nonlethal and lethal metatropic dysplasia. Am J Med Genet A 152A:1169–1177
Rock MJ, Prenen J, Funari VA et al (2008) Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet 40:999–1003
Everaerts W, Nilius B, Owsianik G (2010) The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 103:2–17
Lamandé SR, Yuan Y, Gresshoff IL et al (2011) Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat Genet 43(11):1142–1146
D'hoedt D, Owsianik G, Prenen J, Cuajungco MP, Grimm C, Heller S, Voets T, Nilius B (2008) Stimulus-specific modulation of the cation channel TRPV4 by PACSIN 3. J Biol Chem 283:6272–6280
Unger S, Lausch E, Stanzial F et al (2011) Fetal akinesia in metatropic dysplasia: the combined phenotype of chondrodysplasia and neuropathy? Am J Med Genet 11:155A2860–155A2864
Fecto F, Shi Y, Huda R, Martina M, Siddique T, Deng HX (2011) Mutant TRPV4-mediated toxicity is linked to increased constitutive function in axonal neuropathies. J Biol Chem 286:17281–17291
Jurkat-Rott K, Holzherr B, Fauler M, Lehmann-Horn F (2010) Sodium channelopathies of skeletal muscle result from gain or loss of function. Pflugers Arch 460:239–248
Sevilla T, Cuesta A, Chumillas MJ, Mayordomo F, Pedrola L, Palau F, Vílchez JJ (2003) Clinical, electrophysiological and morphological findings of Charcot–Marie–Tooth neuropathy with vocal cord palsy and mutations in the GDAP1 gene. Brain 126:2023–2033
Pareyson D, Taroni F, Botti S, Morbin M, Baratta S, Lauria G, Ciano C, Sghirlanzoni A (2000) Cranial nerve involvement in CMT disease type 1 due to early growth response 2 gene mutation. Neurology 54:1696–1698
Puls I, Jonnakuty C, LaMonte BH et al (2003) Mutant dynactin in motor neuron disease. Nat Genet 33:455–456
McEntagart M, Norton N, Williams H et al (2001) Localization of the gene for distal hereditary motor neuronopathy VII (dHMN-VII) to chromosome 2q14. Am J Hum Genet 68:1270–1276
Pareyson D, Marchesi C, Salsano E (2009) Hereditary predominantly motor neuropathies. Curr Opin Neurol 22:451–459
Liedtke W, Choe Y, Marti-Renom MA et al (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535
Amato V, Viña E, Calavia MG et al (2012) TRPV4 in the sensory organs of adult zebrafish. Microsc Res Tech 75(1):89–96
Simons KT, Kooperberg C, Huang E, Baker D (1997) Assembly of protein tertiary structures from fragments with similar local sequences using simulated annealing and Bayesian scoring functions. J Mol Biol 268:209–225
Rohl CA, Strauss CE, Chivian D, Baker D (2004) Modeling structurally variable regions in homologous proteins with rosetta. Proteins 55:656–677
Bonneau R, Strauss CE, Rohl CA et al (2002) De novo prediction of three-dimensional structures for major protein families. J Mol Biol 322:65–78
Acknowledgments
We thank Mrs. Catherine Wrenn for her valuable help in the English writing. This study was supported in part by the Italian Ministry of Health, Regione Toscana grant RR5/09-RT (to CB) and Fondazione Telethon (Grant GUP08005 to CB).
Ethical standards
The authors declare that all the experiments and clinical studies comply with the current laws of the country in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Immunofuorescence (IF) panel of muscle and skin sections from a control and patient 1 to evaluate the expression of the TRPV4 gene product. In muscle sections from both patient and control, TRPV4 is diffusely but modestly expressed in the cytosol (A, C). In skin sections (B, D) TRPV4 shows intense binding on keratinocyte layer, endothelium and nerve endings, as described [11, 22]. No significant differences can be observed between patient and control. (JPEG 83 kb)
Supplementary Figure 2
Expression of the TRPV4 gene product in cultured skin fibroblasts from control, patients 1 and 2. TRPV4 has intense staining in the nucleus as described by the antibody manufacturer. No differences can be seen between the two patients in respect to control. (JPEG 51 kb)
Supplementary Figure 3
Structural representation of the N-terminus of the TRPV4 protein is presented. (A) Ribbon representation of one of the structural models obtained for the N-terminal region (Met1-Thr399) of TRPV4. The structural prediction was performed using the protein structure prediction server Robetta [24, 25]. The first 130 N-terminal residues, without a detectable PDB homolog, were modeled using the Rosetta de novo approach [26], while comparative models for the ankyrin-repeat domain (ARD) were built using the PDB structure of the chicken TRPV4 ARD as template [3]. The ARD with six putative repeat units, the proline-rich domain and the N-terminal 1–131 domain are depicted in red, orange and green, respectively. Residues Pro97 (in blue) and Arg232 (in cyan) are shown. (B) Modeling of the NH2-terminus region (residues 1–470) with I-TASSER server (http://zhang.bioinform-atics.ku.edu/I-TASSER/). Visualization and molecular graphic of the final model was rendered using Chimera (http://www.cgl.ucsf.edu/chimera/). The ribbon representation shows the six ankyrin repeats (ANK; blue), the proline-rich domain in yellow and the P97 and R232 residues, indicated by arrows with dotted lines. (JPEG 44 kb)
Rights and permissions
About this article
Cite this article
Fiorillo, C., Moro, F., Brisca, G. et al. TRPV4 mutations in children with congenital distal spinal muscular atrophy. Neurogenetics 13, 195–203 (2012). https://doi.org/10.1007/s10048-012-0328-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-012-0328-7