Abstract
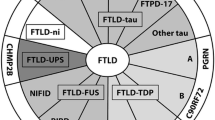
The past year has seen a number of significant advances in our understanding of the neuropathological and molecular genetic basis of frontotemporal lobar degeneration (FTLD). Whereas, in the past, most attention focused on FTLD associated with tau-based pathology and microtubule associated protein tau gene (MAPT) mutations, there has recently been greater attention paid to non-tau FTLD. FTLD with tau-negative, ubiquitinated inclusions (FTLD-U) is now recognized as the most common pathology associated with clinical FTLD. Mutations in the progranulin gene (PGRN) have been identified as the cause of FTLD-U linked to chromosome 17. A rapidly growing number of PGRN mutations have been identified, and to date, all appear to cause FTLD by reducing the amount of functional PGRN protein (haploinsufficiency). The neuropathology associated with each of the known non-MAPT FTLD genes and loci (PGRN, valosin-containing protein gene, CHMP2B and 9p), has been shown to be a specific subtype of FTLD-U. The ubiquitinated pathological protein in FTLD-U has been identified as TAR deoxyribonucleic acid-binding protein with M r 43 kDa (TDP-43). Immunohistochemical and biochemical studies of TDP-43 have helped to clarify the relationship between different sub-types of FTLD-U and related conditions. It is anticipated that these discoveries will facilitate the development of new diagnostic tests and therapeutics.






Similar content being viewed by others
References
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Bird T, Knopman D, VanSwieten J, Rosso S, Feldman H, Tanabe H, Graff-Raford N, Geschwind D, Verpillat P, Hutton M (2003) Epidemiology and genetics of frontotemporal dementia/Pick's disease. Ann Neurol 54(Suppl 5):S29–S31
Feldman H, Levy AR, Hsiung GY, Peters KR, Donald A, Black SE, Bouchard RW, Gauthier SG, Guzman DA, Hogan DB, Kertesz A, Rockwood K (2003) A Canadian Cohort Study of Cognitive Impairment and Related Dementias (ACCORD): study methods and baseline results. Neuroepidemiology 22:265–274
Chow TW, Miller BL, Hayashi VN, Geschwind DH (1999) Inheritance of frontotemporal dementia. Arch Neurol 56:817–822
Rosso SM, Donker KL, Baks T, Joosse M, de Koning I, Pijnenburg Y, de Jong D, Dooijes D, Kamphorst W, Ravid R, Niermeijer MF, Verheij F, Kremer HP, Scheltens P, van Duijn CM, Heutink P, van Swieten JC (2003) Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain 126:2016–2022
Stevens M, van Duijn CM, Kamphorst W, de Knijff P, Heutink P, van Gool WA, Scheltens P, Ravid R, Oostra BA, Niermeijer MF, van Swieten JC (1998) Familial aggregation in frontotemporal dementia. Neurology 50:1541–1545
Lomen-Hoerth C, Anderson T, Miller B (2002) The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 59:1077–1079
Trojanowski JQ, Dickson D (2001) Update on the neuropathological diagnosis of frontotemporal dementia. J Neuropathol Exp Neurol 60:1123–1126
Knopman DS, Mastri AR, Frey WH, Sung JH, Rustan T (1990) Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology 40:251–256
Okamoto K, Murakami N, Kusaka H, Yoshida M, Hashizume Y, Nakazato Y, Matsubara E, Hirai S (1992) Ubiquitin-positive intraneuronal inclusions in the extramotor cortices of presenile dementia patients with motor neuron disease. J Neurol 239:426–430
Wightman G, Anderson VER, Martin J, Swash M, Anderton BH, Neary D, Mann D, Luthert P, Leigh PN (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Jackson M, Lennox G, Lowe J (1996) Motor neurone disease-inclusion dementia. Neurodegeneration 5:339–350
Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, Chute DJ, Roberson ED, Pace-Savitsky C, Neumann M, Chow TW, Rosen HJ, Forstl H, Kurz A, Miller BL (2005) Frontotemporal lobar degeneration. Demographic characteristics of 353 patients. Arch Neurol 62:925–930
Jospehs KA, Holton JL, Rossor MN, Godbolt AK, Ozawa T, Strand K, Khan N, Al-Sarraj S, Revesz T (2004) Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 30:369–373
Mackenzie IRA, Shi J, Shaw CL, DuPlessis D, Neary D, Snowden JS, Mann DMA (2006) Dementia Lacking Distinctive Histology (DLDH) revisited. Acta Neuropathol 112:539–549
Hutton M, Lendon C, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, Van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, Van Swieten J, Mann D, Lynch T, Heutink P (1998) Coding and 5′ splice mutations in tau associated with inherited dementia (FTDP-17). Nature 393:702–705
Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43:815–826
Spillantini MG, Murrell JR, Goedert M, Farlow MB, Klug A, Ghetti B (1998) Mutation in the tau gene in familial multisystem tauopathy with presenile dementia. Proc Natl Acad Sci USA 95:7737–7741
Rademakers R, Cruts M, van Broeckhoven C (2004) The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 24:277–295
Buee L, Delacourte A (1999) Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol 9:681–693
Mackenzie IRA, Baborie A, Pickering-Brown S, du Pleissis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DMA (2006) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112:539–549
Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VMY (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352
Poorkaj P, Grossman M, Steinbart E, Payani H, Sadovnick A, Nochlin D, Tabira T, Trojanowski JQ, Borson S, Galasko D, Reich S, Quinn B, Schellenberg G, Bird TD (2001) Frequency of taugene mutations in familial and sporadic cases of non-Alzheimer’s dementia. Arch Neurol 58:383–387
Ghetti B, Hutton ML, Wszolek ZK (2003) Frontotemporal dementia and parkinsonism linked to chromosome 17 associated with Tau gene mutations (FTDP-17T). In: Dickson D (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath, Basal, pp 86–102
Benussi L, Signorini S, Ghidoni R, Alberici A, Barbiero L, Feudatari E, Gigola L, Nicosia F, Paterlini A, Strom T, Bettecken T, Meitinger T, Binetti G (2004) Identification of genetic loci associated with familial frontotemporal dementia. Neurobiol Aging 25:S517–S518
Bird TD, Wijsman EM, Nochlin D, Leehey M, Sumi SM, Payami H, Poorkaj P, Nemens E, Rafkind M, Schellenberg GD (1997) Chromosome 17 and hereditary dementia: linkage studies in three non-Alzheimer families and kindreds with late-onset FAD. Neurology 48:949–954
Froelich S, Basun H, Forsell C, Lilius L, Axelman K, Andreadis A, Lannfelt L (1997) Mapping of a disease locus for familial rapidly progressive frontotemporal dementia to chromosome 17q12-21. Am J Med Genet 74:380–385
Kertesz A, Kawarai T, Rogaeva E, St George-Hyslop P, Poorkaj P, Bird TD, Munoz DG (2000) Familial frontotemporal dementia with ubiquitin-positive, tau-negative inclusions. Neurology 54:818–827
Lendon CL, Lynch T, Norton J, McKeel DW, Busfield F, Craddock N, Chakraverty S, Gopalakrishnan G, Shears SD, Grimmett W, Wilhelmsen KC, Hansen L, Morris JC, Goate AM (1998) Hereditary dysphasic disinhibition dementia: a frontotemporal dementia linked to 17q21-22. Neurology 50:1546–1555
Mackenzie IR, Baker M, West G, Woulfe J, Qadi N, Gass J, Cannon A, Adamson J, Feldman H, Lindholm C, Melquist S, Pettman R, Sadovnick AD, Dwosh E, Whiteheart SW, Hutton M, Pickering-Brown SM (2006) A family with tau-negative frontotemporal dementia and neuronal intranuclear inclusions linked to chromosome 17. Brain 129:853–867
Rademakers R, Cruts M, Dermaut B, Sleegers K, Rosso SM, Van den Broeck M, Backhovens H, van Swieten J, van Duijn CM, Van Broeckhoven C (2002) Tau-negative frontal lobe dementia at 17q21: Significant fine mapping of the candidate region to a 4.8-cm interval. Mol Psych 7:1064–1074
Rosso SM, Kamphorst W, deGraaf B, Willemsen R, Ravid R, Niermeijer MF, Spillantini MG, Heutink P, van Swieten JC (2001) Familial frontotemporal dementia with ubiquitin-positive inclusions linked to chromosome 17q21-22. Brain 124:1948–1957
Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Tobinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wills H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, de Pooter T, Mattheijssens M, van den Broeck M, Cuijt I, Vennekens K, de Deyn PP Kumar-Singh S, van Broeckhoven C (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442:920–924
Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementias caused by mutant valosin-containing protein. Nat Genet 36:377–381
Momeni P, Schymick J, Jain S, Cookson MR, Cairns NJ, Greggio E, Greenway MJ, Berger S, Pickering-Brown S, Shio A, Fung HC, Holtzman DM, Huey ED, Wassermann EM, Adamson J, Hutton ML, Rogaeva E, St. George-Hyslop P, Rothstein JD, Hardiman O, Grafman J, Singleton A, Hardy J, Traynor BJ (2006) Analysis of IFT74 as a candidate gene for chromosome 9p-linked ALS-FTD. BMC Neurol 6:44
Morita M, Al Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, Mitchell JE, Habgood JJ, de Belleroche J, Xi J, Jongjaroenprasert W, Horvitz HR, Gunnarsson LG, Brown RH (2006) A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66:839–844
Valdmanis PN, Dupre N, Bouchard JP, Camu W, Meininger V, Strong M, Rouleau GA (2007) Three families with amyotrophic lateral sclerosis and frontotemporal dementia with evidence of linkage to chromosome 9p. Arch Neurol 64:240–245
Vance C, Al-Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, Siddique T, Schelhaas HJ, Kusters B, Troost D, Baas F, de Jong V, Shaw CE (2006) Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain 129:868–876
Hosler BA, Siddique T, Sapp PC, Sailor W, Huang MC, Hossain A, Daube JR, Nance M, Fan C, Kaplan J, Hung WY, McKenna-Yasek D, Haines JL, Pericak-Vance MA, Horvitz HR, Brown RH (2000) Linkage of familial amyotrophic lateral sclerosis with frontotemporal dementia to chromosome 9q21-22. J Am Med Assoc 284:1664–1669
Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sorensen SA, Gydesen S, Fisher EMC, Collinge J (2005) Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 37:806–808
Daniel R, He Z, Carmichael P, Halper J, Bateman A (2000) Cellular localization of gene expression for progranulin. J Histochem Cytochem 48:999–1009
Ahmed Z, Mackenzie I, Hutton M, Dickson D (2007) Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflamm 4:7
He Z, Bateman A (2003) Progranulin (granulin–epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med 81:600–612
Maquat LE (2004) Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat Rev 5:89–99
Le Ber I, van der Zee J, Hannequin D, Gijselinck I, Campion D, Puel M, Laquerriere A, De Pooter T, Camuzat A, Van den Broeck M, Dubois B, Sellal F, Lacomblez L, Vercelletto M, Thomas-Anterion C, Mickel BF, Golfier V, Didic M, Salachas F, Duyckaerts C, Cruts M, Verpillat P, Van Broeckhoven C, Brice A (2007) Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat 28:846–855
Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R, Josephs K, Graff-Radford N, Dickson D, Wszolek Z, Gonzales J, Beach T, Bigio E, Mesalum M, White C, Feldman H, Knopman D, Hutton M, Rademakers R (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15:2988–3001
Mukherjee O, Pastor P, Cairns NJ, Chakraverty S, Kauwe JSK, Shears S, Behrens MI, Budde J, Hinrichs AL, Norton J, Levitch D, Taylor-Reinwald L, Gitcho M, Tu PH, Grinberg LT, Liscic RM, Armendariz J, Morris JC, Goate AM (2006) HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol 60:314–322
Bronner IF, Rizzu P, Seelaar H, van Mil SE, Anar B, Azmani A, Kaat LD, Rosso S, Heutink P, van Swieten JC (2007) Progranulin mutations in Dutch familial frontotemporal lobar degeneration. Eur J Hum Genet 15:369–374
Schmyick J, Yang Y, Andersen P, Vonsattel J, Greenway M, Momeni P, Elder J, Chlo A, Restagno G, Robberecht W, Dahlberg C, Mukherjee O, Goate A, Graff-Radford N, Caselli R, Hutton M, Gass J, Cannon A, Rademakers R, Singleton A, Hardiman O, Rothstein J, Hardy J, Traynor B (2007) Progranulin mutations and ALS or ALS-FTD phenotypes. J Neurol Neurosurg Psychiatry 78:754–756
Spina S, Murrell JR, Huey ED, Wassermann EM, Pietrini P, Baraibar MA, Barbeito AG, Troncoso JC, Vidal R, Ghetti B, Grafman J (2007) Clinicopathologic features of frontotemporal dementia with progranulin sequence variation. Neurology 68:820–827
Van der Zee J, Le Ber I, Maurer-Stroh S, Engelborghs S, Gijselinck I, Camuxat A, Brouwers N, Vandenberghe R, Sleegers K, Hannequin D, Dermaut B, Schymkowitz J, Campion D, Santens P, Martin JJ, Lacomblez L, de Pooter T, Teeters K, Mattheijssens M, Vercelletto M, Van den Broeck M, Cruts M, De Deyn PP, Rouseau F, Brice A, Van Broeckhoven C (2007) Mutations other than null mutations producing a pathogenic loss of progranulin in frontotemporal dementia. Hum Mutat 28:416
Josephs KA, Ahmed Z, Katsuse O, Parisi JF, Boeve BF, Knopman DS, Petersen RC, Davies P, Duara R, Graff-Radford NR, Uitti RJ, Rademakers R, Adamson J, Baker M, Hutton ML, Dickson DW (2007) Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 66:142–151
Masellis M, Momeni P, Meschino W, Heffner R, Elder J, Sato C, Liang, St. George-Hyslop P, Hardy J, Bilbao J, Black S, Rogaeva E (2006) Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain 129:3115–3123
Snowden JS, Pickering-Brown SM, Mackenzie IM, Richardson AMT, Varma A, Neary D, Mann DMA (2006) Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain 129:3091–3102
Mesulam M, Johnson N, Krefft TA, Gass JM, Cannon AD, Adamson JL, Bigio EH, Weintraub S, Dickson DW, Hutton ML, Graff-Radford NR (2007) Progranulin mutations in primary progressive aphasia: the PPA1 and PPA3 families. Arch Neurol 64:43–47
Benussi L, Binetti G, Sina E, Gigola L, Bettecken T, Meitinger T, Ghidoni R (2007) A novel deletion in progranulin gene is associated with FTDP-17 and CBS. Neurobiol Aging (in press), DOI 10.1016/j.neurobiolaging.2006.10.028
Huey ED, Grafman J, Wassermann EM, Pietrini P, Tierney MC, Ghetti B, Spina S, Baker M, Hutton M, Elder JW, Berger SL, Heflin KA, Hardy J, Momeni P (2006) Characteristics of frontotemporal dementia patients with progranulin mutation. Ann Neurol 60:374–380
Leverenz JB, Yu CE, Montine TJ, Steinbart E, Bekris LM, Zabetian C, Kwong LK, Lee VMY, Schellenberg GD, Bird TD Leverenz JB, Yu CE, Montine TJ et al (2007) A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain 130:1360–1374
Mackenzie IRA, Baker M, Pickering-Brown S, Hsiung GYR, Lindholm C, Dwosh E, Gass J, Cannon A, Rademakers R, Hutton M, Feldman HH (2006) The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129:3081–3090
Woulfe J, Kertesz A, Munoz DG (2001) Frontotemporal dementia with ubiquitinated cytoplasmic and intranuclear inclusions. Acta Neuropathol 102:94–102
Mackenzie IRA, Feldman H (2003) Neuronal intranuclear inclusions distinguish familial FTD-MND type from sporadic cases. Acta Neuropathol 105:543–548
Bigio EH, Johnson NA, Rademaker AW, Fung BB, Mesulam MM, Siddique N, Dellefave L, Caliendo J, Freeman S, Siddique T (2004) Neuronal ubiquitinated intranuclear inclusions in familial and non-familial frontotemporal dementia of the motor neuron disease type associated with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 63:801–811
Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, Jhaveri BS, Karlawish JH, Pestronk A, Smith TW, Tu PH, Watts GDJ, Markesbery WR, Smith CD, Kimonis VE (2006) Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol 65:571–581
Schroder R, Watts GDJ, Mehta SG, Evert BO, Broich P, Fliesbach K, Pauls K, Hans VH, Kimonis V, Thal DR (2005) Mutant valosin-containing protein causes a novel type of frontotemporal dementia. Ann Neurol 57:457–461
Kovach MJ, Waggoner B, Leal SM, Gelber D, Khardori R, Levenstien MA, Shanks CA, Gregg G, Al Lozi MT, Miller T, Rakowicz W, Lopate G, Florence J, Glosser G, Simmons Z, Morris JC, Whyte MP, Pestronk A, Kimonis VE (2001) Clinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget’s disease of bone and frontotemporal dementia. Mol Genet Metab 74:458–475
Wang Q, Song C, Li CC (2004) Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol 146:44–57
Gydesen S, Brown JM, Brun A, Chakrabarti L, Gade A, Johannsen P, Rossor M, Thusgaard T, Grove A, Yancopoulou D, Spillantini MG, Fisher EM, Collinge J, Sorensen SA (2002) Chromosome 3 linked frontotemporal dementia (FTD-3). Neurology 59:1585–1594
Holm IE, Englund E, Mackenzie IRA, Johannsen P, Isaacs A (2007) A reassessment of the neuropathology of frontotemporal dementia linked to chromosome 3 (FTD-3). J Neuorpathol Exp Neurol (in press)
Goldstein LS, Philip AV (1999) The road les traveled: emertging principles of kinesin mortor utilization. Annu Rev Cell Dev Biol 15:141–183
Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, Skehel P, Zatz M (2004) A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet 75:822–831
Schmitt-John T, Drepper C, Mussmann A, Hahn P, Kuhlmann M, Thiel C, Hafner M, Lengeling A, Heimann P, Jones JM, Meisler MH, Jockusch H (2005) Mutation of Vsp54 causes motor neuron disease and defective spermiognesis in the wobbler mouse. Nat Genet 37:1213–1215
Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, Schneider JA, Carter E, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VMY, Mackenzie IRA (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VMY (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB (1995) Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol 69:3584–3596
Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE (2001) Nuclear factor TDP-43 and SP proteins promote in vitro and in vivo CFTR exon skipping. EMBO J 20:1774–1784
Wang HY, Wang IF, Bose J, Shen CK (2004) Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics 83:130–139
Mercado PA, Ayala YM, Romano M, Buratti E, Baralle FE (2005) Depletion of TDP43 overrides the need for exonic and intronic splicing enhancers in the human apoA-II gene. Nucleic Acids Res 33:6000–6010
Mackenzie IRA, Feldman H (2005) Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol 64:730–739
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Davidson Y, Kelley T, Mackenzie IRA, Pickering-Brown S, Du Plessis D, Neary D, Snowden JS, Mann DMA (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533
Neumann M, Kwong LK, Traux AC, Vanmassenhove B, Kretzschmar HA, Van Deerlin VM, Clark CM, Grossman M, Miller BL, Trojanowski JQ, Lee VMY (2007) TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol 66:177–183
Mackenzie IRA, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley RJ, Kirby J, Siddique T, Shaw PJ, Lee VMY, Trojanowski JQ (2007) Pathological TDP-43 distinguishes sporadic ALS from ALS with SOD-1 mutations. Ann Neurol 61:427–434
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Rollinson S, Snowden JS, Neary D, Morrison KE, Mann DM, Pickering-Brown SM (2007) TDP-43 gene analysis in frontotemporal lobar degeneration. Neurosci Lett 419:1–4
Schumacher A, Friedrich P, Diehl-Schmid J, Ibach B, Perneczky R, Eisele T, Vukovich R, Foerstl H, Riemenschneider M (2007) No association of TDP-43 with sporadic frontotemporal dementia. Neurobiol Aging (in press), DOI 10.1016/j.neurobiologyaging.2007.05.022
Acknowledgements
We would like to thank the families who contributed samples that were critically important to past, present and future research. The immunoblot image for Fig. 6 was generously provided by Linda Kwong, University of Pennsylvania. Our work was supported by the Pacific Alzheimer Research Foundation, CIHR (grant no. 74580), NIH (P01 AG017216, R01 AG026251, P50 AG16574), The Mayo Clinic Research Foundation and the Robert and Clarice Smith Fellowship programme. RR is a post-doctoral fellow of the Fund for Scientific Research Flanders (FWO-F).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mackenzie, I.R.A., Rademakers, R. The molecular genetics and neuropathology of frontotemporal lobar degeneration: recent developments. Neurogenetics 8, 237–248 (2007). https://doi.org/10.1007/s10048-007-0102-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-007-0102-4