Abstract
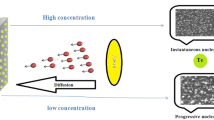
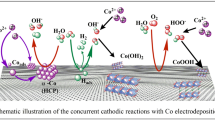
A comparative study of the electrochemical deposition of cobalt on tin-doped indium oxide (ITO) substrate was carried out using electrochemical techniques in nitrate and chloride electrolytes. The bath used for the study in a nitrate medium contains 0.01 M of cobalt nitrate hexahydrate (Co(NO3)2. 6H2O) with 0.1 M of potassium nitrate (KNO3). For the chloride medium study, the electrolyte consists of a mixture of 0.01 M CoCl2 with 0.1 M potassium chloride (KCl). The cyclic voltammetry (CV) and the chronoamperometry (CA) have been used to study the kinetics, nucleation, and growth mechanism. The (CV) and (CA) measurements revealed that the electrodeposition of cobalt (Co) at a negative potential around −0.95 V versus SCE (saturated calomel electrode) is a quasi-reversible reaction controlled by the diffusion process in the two electrolytes. The measured current transient curves were compared to those calculated from Scharifker-Hills and Heerman-Tarallo models. It was found that a progressive three-dimensional (3D) nucleation mechanism governed the nucleation and the growth of Co on the ITO substrate in chloride solution, while in nitrate solution, the nucleation mechanism followed the instantaneous 3D model. The characterization of samples by X-ray diffraction had shown that the cobalt electrodeposited on ITO substrate has a hexagonal crystal structure whatever the electrolyte composition.









Similar content being viewed by others
References
Lghazi Y, Moubah R, Bimaghra I, Hlil Ek, ElBahraoui T, Allegand S, Lasri H (2017) Investegation of structural and magnetic properties in electrodeposed Co/CoxZn1-x superlattices. J Supercond Nov Magn 30(9):2641–2645. https://doi.org/10.1007/s10948-017-4075-8
Lee KK, Loh PY, Sow CH, Chin WS (2012) CoOOH nanosheets on cobalt substrate as a non-enzymatic glucose sensor. Electrochem Commun 20:128–132. https://doi.org/10.1016/j.elecom.2012.04.012
Chen M, Xia X, Zhang J, Qi M, Yin J, Chen Q (2016) Controllable synthesis of cobalt oxide nanoflakes on three-dimensional porous cobalt networks as high-performance cathode for alkaline hybrid batteries. Mater Res Bull 74:472–477. https://doi.org/10.1016/j.materresbull.2015.11.025
Yang Y, Fei H, Ruan G, Tour JM (2015) Porous cobalt-based thin film as a bifunctional catalyst for hydrogen generation and oxygen generation. Adv Mater 27(20):3175–3180. https://doi.org/10.1002/adma.201500894
Zhang L, Yang C, Zhao G, Mu J, Wang Y (2015) Self-supported porous CoOOH nanosheet arrays as a non-enzymatic glucose sensor with good reproducibility. Sensors Actuators B Chem 210:190–196. https://doi.org/10.1016/j.snb.2014.12.113
Wang X, Tian W, Zhai T, Zhi C, Bando Y, Golberg D (2012) Cobalt(II,III) oxide hollow structures: fabrication, properties and applications. J Mater Chem 22(44):23310–23326. https://doi.org/10.1039/c2jm33940d
Jiang J, Liu J, Ding R, Zhu J, Li Y, Hu A, Li X, Huang X (2011) Large-scale uniform α-Co(OH)2 long nanowire arrays grown on graphite as pseudocapacitor electrodes. ACS Appl Mater Interfaces 3(1):99–103. https://doi.org/10.1021/am1009887
Hou Y, Kondoh H, Shimojo M, Kogure T, Ohta T (2005) High-yield preparation of uniform cobalt hydroxide and oxide nanoplatelets and their characterization. J Phys Chem B 109(41):19094–19098. https://doi.org/10.1021/jp0521149
Rios-Reyes CH, Mendoza-Huizar LH, Rivera M (2009) Electrochemical kinetic study about cobalt electrodeposition onto GCE and HOPG substrates from sulfate sodium solutions. J Solid State Electrochem 14(4):659–668. https://doi.org/10.1007/s10008-009-0816-3
Ait Himi M, El Ghachtouli S, Amarray A et al (2020) Removal of azo dye Calcon using polyaniline films electrodeposited on SnO2 substrate. Phys Chem Res 8:111–124. https://doi.org/10.22036/pcr.2019.203023.1680
Miranda-Hernández M, Palomar-Pardavé M, Batina N, González I (1998) Identification of different silver nucleation processes on vitreous carbon surfaces from an ammonia electrolytic bath. J Electroanal Chem 443(1):81–93. https://doi.org/10.1016/S0022-0728(97)00487-7
Fletcher S, Halliday CS, Gates D, Westcott M, Lwln T, Nelson G (1983) The response of some nucleation/growth processes to triangular scans of potential. J Electroanal Chem Interfacial Electrochem 159(2):267–285. https://doi.org/10.1016/S0022-0728(83)80627-5
Mendoza-Huizar LH, Robles J, Palomar-Pardavé M (2003) Nucleation and growth of cobalt onto different substrates - Part II. The upd-opd transition onto a gold electrode. J Electroanal Chem 545:39–45. https://doi.org/10.1016/S0022-0728(03)00087-1
Bard AJ, Faulkner LR (1980) Electrochemical method: fundamentals and applications. Wiley, New York 91-92 and 500-511
Zhang C, Jiang L, Xu F, Duan N, Xin B, Han G, Zhang G, Wen Y (2018) New insight into cleaner control of heavy metal anode slime from aqueous sulfate electrolytes containing Mn (II): preliminary characterization and mechanism analysis. J Clean Prod 177:276–283. https://doi.org/10.1016/j.jclepro.2017.12.252
Mashreghi A, Zare H (2016) Investigation of nucleation and growth mechanism during electrochemical deposition of nickel on fluorine doped tin oxide substrate. Curr Appl Phys 16(5):599–604. https://doi.org/10.1016/j.cap.2016.03.008
Ait Himi M, El Ghachtouli S, Youbi B et al (2019) Nucleation and growth mechanism of manganese oxide electrodeposited on ITO substrate. Mater Today Proc 30:963–969. https://doi.org/10.1016/j.matpr.2020.04.358
Gupta A, Srivastava C (2020) Nucleation and growth mechanism of tin electrodeposition on graphene oxide: a kinetic, thermodynamic and microscopic study. J Electroanal Chem 861:113964. https://doi.org/10.1016/j.jelechem.2020.113964
Ait Ahmed N, Eyraud M, Hammache H, Vacandio F, Sam S, Gabouze N, Knauth P, Pelzer K, Djeniziand T (2013) New insight into the mechanism of cathodic electrodeposition of zinc oxide thin films onto vitreous carbon. Electrochim Acta 94:238–244. https://doi.org/10.1016/j.electacta.2013.01.103
Youbi B, Lghazi Y, Ait Himi M et al (2020) Growth mechanism during the early stages of electrodeposition of bismuth telluride Bi2Te3 on ITO substrate. Mater Today Proc 30:842–848. https://doi.org/10.1016/j.matpr.2020.04.338
Lghazi Y, Bimaghra I, El Bachiri A, Elmerzouki K, Youbi B, Lasri H (2018) Investigation of the nucleation kinetics of Bi and Δ-Bi2O3 during electro-deposition on substrate ITO. Int J Eng Technol 7(4.32):21–24
Astley DJ, Harrison JA, Thirsk HR (1968) Electrocrystallization of mercury, silver and palladium. Trans Faraday Soc 64:192. https://doi.org/10.1039/TF9686400192
Gunawardena G, Hills G, Scharifker B (1981) Induction times for the formation of single mercury nuclei on a platinum microelectrode. J Electroanal Chem 130:99–112. https://doi.org/10.1016/S0022-0728(81)80379-8
Youbi B, Lghazi Y, El Bachiri A et al (2020) Investigation of nucleation and growth mechanism of bismuth electrodeposited on ITO substrate in nitric acid medium. Mater Today Proc 22:6–11. https://doi.org/10.1016/j.matpr.2019.08.055
Youbi B, Lghazi Y, Ait Himi M, Bimaghra I (2019) Nucleation and growth mechanism of tellurium electrodeposited on tin-doped indium oxide substrate. J Appl Electrochem 50(2):159–168. https://doi.org/10.1007/s10800-019-01377-0
Mezine Z, Kadri A, Hamadou L, Benbrahim N, Chaouchi A (2018) Electrodeposition of copper oxides (CuxOy) from acetate bath. J Electroanal Chem 817:36–47. https://doi.org/10.1016/j.jelechem.2018.03.055
Scharifker B, Hills G (1983) Theoretical and experimental studies of multiple nucleation. Electrochim Acta 28(7):879–889. https://doi.org/10.1016/0013-4686(83)85163-9
Heerman L, Tarallo A (1999) Theory of the chronoamperometric transient for electrochemical nucleation with diffusion-controlled growth. J Electroanal Chem 470(1):70–76. https://doi.org/10.1016/S0022-0728(99)00221-1
Alvarez AE, Salinas DR (2010) Formation of Cu/Pd bimetallic crystals by electrochemical deposition. Electrochim Acta 55(11):3714–3720. https://doi.org/10.1016/j.electacta.2010.01.076
Sluyters-Rehbach M, Wijenberg JHOJ, Bosco E, Sluyters JH (1987) The theory of chronoamperometry for the investigation of electrocrystallization. Mathematical description and analysis in the case of diffusion-controlled growth. J Electroanal Chem 236(1-2):1–20. https://doi.org/10.1016/0022-0728(87)88014-2
Scharifker B, Mostany J (1984) Three-dimensional nucleation with diffusion controlled growth Part I. Number density of active sites and nucleation rates per site. J Electroanal Chem 177(1-2):13–23. https://doi.org/10.1016/0022-0728(84)80207-7
Heerman L, Tarallo A (1998) Electrochemical nucleation on microelectrodes. Theory and experiment for diffusion-controlled growth. J Electroanal Chem 451(1-2):101–109. https://doi.org/10.1016/S0022-0728(98)00101-6
Palomar-Pardavé M, Scharifker BR, Arce EM, Romero-Romo M (2005) Nucleation and diffusion-controlled growth of electroactive centers: reduction of protons during cobalt electrodeposition. Electrochim Acta 50(24):4736–4745. https://doi.org/10.1016/j.electacta.2005.03.004
Deutscher RL, Fletcher S (1988) Nucleation on active sites. Part IV. Invention of an electronic method of counting the number of crystals as a function of time; and the discovery of nucleation rate dispersion. J Electroanal Chem 239(1-2):17–54. https://doi.org/10.1016/0022-0728(88)80268-7
Yuan X, Hu XX, Ding XL, Kong HC, Sha HD, Lin H, Wen W, Shen G, Guo Z, Ma ZF, Yang Y (2013) Effects of cobalt precursor on pyrolyzed carbon-supported cobalt-polypyrrole as electrocatalyst toward oxygen reduction reaction. Nanoscale Res Lett 8(1):478. https://doi.org/10.1186/1556-276X-8-478
Premlatha S, Bapu GNKR (2018) Direct current electrodeposition of Co-ITO nanoflakes modified steel electrode for highly selective non enzymatic detection of catechol. J Alloys Compd 767:622–631. https://doi.org/10.1016/j.jallcom.2018.07.119
Kidosaki T, Takase S, Shimizu Y (2012) Electrodeposited cobalt-iron alloy thin-film for potentiometric hydrogen phosphate-ion sensor. J Sens Technol 2(03):95–101. https://doi.org/10.4236/jst.2012.23014
Monshi A, Foroughi MR, Monshi MR (2012) Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J Nano Sci Eng 02(03):154–160. https://doi.org/10.4236/wjnse.2012.23020
Acknowledgements
This work is carried out at Bio-Geosciences and Materials Engineering laboratory at the ENS Casablanca, Morocco. The authors would like to thank all those who helped them to carry out the analysis at the XRD at the faculty of sciences Ain Chock, Casablanca.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bahar, J., Lghazi, Y., Youbi, B. et al. Comparative study of nucleation and growth mechanism of cobalt electrodeposited on ITO substrate in nitrate and chloride electrolytes. J Solid State Electrochem 25, 1889–1900 (2021). https://doi.org/10.1007/s10008-021-04961-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-04961-7