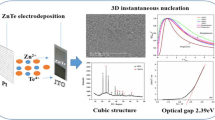
Abstract
The mechanism of electrochemical deposition of tellurium on tin-doped indium oxide (ITO) substrate from a nitric acid solution containing telluryl ion (HTeO2+) has been studied in this work. The investigation, using cyclic voltammetry and chronoamperometry, shows that the electrodeposition of Te at a negative potential around − 0.42 V versus saturated calomel electrode (SCE) is a quasi-reversible reaction controlled by the diffusion process. Furthermore, by chronoamperometry, the measured current transient curves were compared with the theoretical models, proposed by Scharifker–Hills and Heerman–Tarallo models. It was found that the nucleation and growth mechanism of Te on ITO substrate changed with increasing the concentration of HTeO2+, it can be instantaneous for 2.10−2 mol L−1 or progressive for 2.10−3 mol L−1. The quantitative analysis (nonlinear fitting) shows that the diffusion coefficient remains between 7.10−6 and 9.10−6 cm2 s−1, the nucleation rate constant A and the number density of active sites N0 were growth with the increase of the concentration and the deposition potential. The thin tellurium layers, electrodeposited by chronoamperometry, are characterized by X-ray diffraction. It has been identified that all the films were polycrystalline type hexagonal crystal structure with preferred orientation of (1 0 0) and (1 1 0) planes to growth. The SEM analysis reveals that the morphology of the resulting deposits is depending on the type of electrodeposition mechanism.
Graphic abstract















Similar content being viewed by others
References
Abad B, Rull-bravo M, Hodson SL, Xu X, Martin-gonzalez M (2015) Thermoelectric properties of electrodeposited tellurium ilms and the sssssodium lignosulfonate effect. Electrochim Acta 169:37–45. https://doi.org/10.1016/j.electacta.2015.04.063
Gerstenhauer E, Grosse P (1982) Phys A 38:35–38
Royer D, Dieulesaint E, Royer D, Dieulesaint E (2012) Elastic and piezoelectric constants of trigonal selenium and tellurium crystals Elastic and piezoelectric constants of trigonal selenium and tellurium crystals. J Appl Phys 50:4042. https://doi.org/10.1063/1.326485
Waldiya M, Bhagat D, Narasimman R, Singh S, Kumar A, Ray A, Mukhopadhyay I (2019) Development of highly sensitive H2O2 redox sensor from electrodeposited tellurium nanoparticles using ionic liquid. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2019.02.050
Avenue B (1986) Electrodeposited CdSe0.5 Te0.5: photoelectrochemical solar cells. J Appl Electrochem 16:168–174
Photovoltaics I, Crisp RW, Panthani MG, Rance WL, Duenow JN, Parilla PA, Callahan R, Dabney MS, Berry JJ, Talapin DV, Luther JM (2014) Nanocrystal grain growth and device architectures for high-efficiency CdTe ink-based photovoltaics. ACS Nano 8(9):9063–9072
Chang T, Cho S, Kim J, Schoenleber J, Frantz C, Stein N, Boulanger C, Lee W (2015) Individual thermoelectric properties of electrodeposited bismuth telluride nanowires in polycarbonate membranes. Electrochim Acta 161:403–407. https://doi.org/10.1016/j.electacta.2015.02.105
Fan Y, Yang Y, Xiao Y, Zhao Z, Lei Y (2013) Hydrometallurgy recovery of tellurium from high tellurium-bearing materials by alkaline pressure leaching process: thermodynamic evaluation and experimental study. Hydrometallurgy 139:95–99. https://doi.org/10.1016/j.hydromet.2013.07.005
Landolt D (2002) Electrodeposition science and technology in the last quarter of the twentieth century. J Electrochecm Soc 149(3):S9–S20
Zhang C, Jiang L, Xu F, Duan N, Xin B (2018) New insight into cleaner control of heavy metal anode slime from aqueous sulfate electrolytes containing Mn (II): preliminary characterization and mechanism analysis. J Clean Prod 177:276–283. https://doi.org/10.1016/j.jclepro.2017.12.252
Search H, Journals C, Contact A, Iopscience M, Address IP, RUSSIAN CHEMICAL, 235 (n.d.)
Montiel-Santillan T, Solorza O, Sanchez H (2002) Electrochemical research on tellurium deposition from acid sulfate medium. J Solid State Electrochem 6(7):433–442. https://doi.org/10.1007/s10008-002-0268-5
Kowalik R, Kutyła D, Mech K, Żabiński P (2016) Analysis of tellurium thin films electrodeposition from acidic citric bath. Appl Surf Sci 388:817–824. https://doi.org/10.1016/j.apsusc.2016.03.127
Jin W, Hu M, Hu J (2018) Selective and efficient electrochemical recovery of dilute copper and tellurium from acidic chloride solutions. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.8b03150
Fan Y, Jiang L, Yang J, Jiang Y, Liu F (2016) The electrochemical behavior of tellurium on stainless steel substrate in alkaline solution and the illumination effects. JEAC. https://doi.org/10.1016/j.jelechem.2016.03.043
Jeng EG-S (1997) Electrochemistry of tellurium(IV) in the basic aluminum chloride-1-methyl-3-ethylimidazolium chloride room temperature molten salt. J Electrochem Soc 144:2369. https://doi.org/10.1149/1.1837820
Sarala Y, Reddy SJ (1986) Electrochemical reduction of tellurium(IV). J Electroanal Chem 214:179–190. https://doi.org/10.1016/0022-0728(86)80095-X
Traore M, Modolo R, Vittori O (1988) Electrochemical behaviour of tellurium and silver telluride at rotating glassy carbon electrode. Electrochim Acta 33:991–996. https://doi.org/10.1016/0013-4686(88)80100-2
Zhong MGQ (2011) Copper electrocrystallization from acidic sulfate electrolyte containing MPS additive. J Appl Electrochem 41:765–771. https://doi.org/10.1007/s10800-011-0293-0
Lou W, Cai W, Li P, Su J, Zheng S, Zhang Y, Jin W (2017) Additives-assisted electrodeposition of fine spherical copper powder from sulfuric acid solution. Powder Technol. https://doi.org/10.1016/j.powtec.2017.12.060
Berzins T, Delahay P (1953) Oscillographic polarographic waves for the reversible deposition of metals on solid electrodes. Chem Soc 75:555
Yang M, Hu Z (2005) Electrodeposition of bismuth onto glassy carbon electrodes from nitrate solutions. J Electroanal Chem 583:46–55. https://doi.org/10.1016/j.jelechem.2005.04.019
Mostany J, Mozota J, Scharifker BR (1984) Three-dimensional nucleation with diffusion controlled growth. J Electroanal Chem Interfacial Electrochem 177:25–37. https://doi.org/10.1016/0022-0728(84)80208-9
Scharifker B, Hills G (1983) Theoretical and experimental studies of multiple nucleation. Electrochim Acta 28:879–889. https://doi.org/10.1016/0013-4686(83)85163-9
Heerman L, Tarallo A (1999) Theory of the chronoamperometric transient for electrochemical nucleation with diffusion-controlled growth. J Electroanal Chem 470:70–76. https://doi.org/10.1016/S0022-0728(99)00221-1
Cao Y, West AC (2015) Nucleation and three-dimensional growth: deviation from diffusion control. J Electrochem Soc 149(4):223–228. https://doi.org/10.1149/1.1461379
Sluyters-Rehbach M, Wijenberg JHOJ, Bosco E, Sluyters JH (1987) The theory of chronoamperometry for the investigation of electrocrystallization. J Electroanal Chem Interfacial Electrochem 236:1–20. https://doi.org/10.1016/0022-0728(87)88014-2
Heerman L, Tarallo A (1998) Electrochemical nucleation on microelectrodes. Theory and experiment for diffusion-controlled growth. J Electroanal Chem 451:101–109
Acknowledgements
The laboratory members of bio-geosciences and materials engineering at the ENS Casablanca are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Youbi, B., Lghazi, Y., Ait Himi, M. et al. Nucleation and growth mechanism of tellurium electrodeposited on tin-doped indium oxide substrate. J Appl Electrochem 50, 159–168 (2020). https://doi.org/10.1007/s10800-019-01377-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-019-01377-0