Abstract
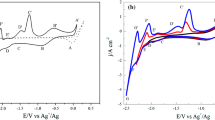
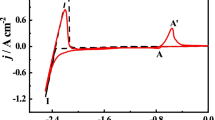
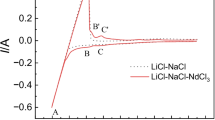
The chloration of MgCl2 was studied in the LiCl–KCl–MgCl2–Gd2O3–Sm2O3 melts. Gd(III) and Sm(III) ions were observed by cyclic voltammetry and square wave voltammetry assisted by MgCl2 in melts. X-ray diffraction (XRD) patterns of melts indicated Gd2O3 and Sm2O3 were chlorinated by MgCl2 and formed GdCl3 and SmCl3. XRD patterns of non-dissolved residues, which were left after the melts were washed with water to remove the soluble salt, showed that the new compounds (i.e., GdOCl, SmOCl, MgO, Gd(OH)3, and Sm(OH)3) were produced. Potentiostatic electrolysis experiments were performed to extract Gd from Gd2O3 and Sm2O3 mixtures assisted by MgCl2. Separation between Gd2O3 and Sm2O3 was also achieved in a single step with the formation of Mg–Li–Gd alloys. XRD patterns of alloys indicated that Mg3Gd phase was formed. Scanning electron microscope images with energy dispersive X-ray spectroscopy showed Gd elements were mainly distributed in the grain boundary.






Similar content being viewed by others
References
Ferry DM, Picard GS, Trémilllon BL (1988) Trans Instn Min Metall 97:C21–C30
Chang YI (1989) Nucl Technol 88:129–131
Sakamura Y, Inoue T, Iwai T, Moriyama H (2005) J Nucl Mater 340:39–51
Tang H, Yan YD, Zhang ML, Li X, Huang Y, Xu YL, Xue Y, Han W, Zhang ZJ (2013) Electrochim Acta 88:457–462
Yan YD, Tang H, Zhang ML, Xue Y, Han W, Cao DX, Zhang ZJ (2012) Electrochim Acta 59:531–537
Bermejo MR, de la Rosa F, Barrado E, Castrillejo Y (2007) J Electroanal Chem 603:81–95
Bermejo MR, Barrado E, Martínez AM, Castrillejo Y (2008) J Electroanal Chem 617:85–100
Bermejo MR, Gómez J, Medina J, Martínez AM, Castrillejo Y (2006) J Electroanal Chem 588:253–266
Cassayre L, Malmbeck R, Masset P, Rebizant J, Serp J, Soucek P, Glatz JP (2007) J Nucl Mater 360:49–57
Castrillejo Y, Bermejo MR, Díaz Arocas P, Martínez AM, Barrado E (2005) J Electroanal Chem 579:343–358
Castrillejo Y, Bermejo MR, Barrado AI, Pardo R, Barrado E, Martínez AM (2005) Electrochim Acta 50:2047–2057
Castrillejo Y, Bermejo MR, Barrado E, Martínez AM (2006) Electrochim Acta 51:1941–1951
Castrillejo Y, Bermejo R, Martínez AM, Barrado E, Díaz Arocas P (2007) J Nucl Mater 360:32–42
Castrillejo Y, Fernández P, Bermejo MR, Barrado E, Martínez AM (2009) Electrochim Acta 54:6212–6222
Castrillejo Y, Fernándeza P, Medina J, Hernández P, Barrado E (2011) Electrochim Acta 56:8638–8644
De Córdoba G, Laplace A, Conocar O, Lacquement J, Caravaca C (2008) Electrochim Acta 54:280–288
Gibilaro M, Massot L, Chamelot P, Taxil P (2008) J Nucl Mater 382:39–45
Gibilaro M, Massot L, Chamelot P, Taxil P (2009) Electrochim Acta 54:5300–5306
Massot L, Chamelot P, Taxil P (2005) Electrochim Acta 50:5510–5517
Sangster J, Pelton AD (1991) J Phase Equilib 12:203
Martínez AM, Børesen B, Haarberg GM, Castrillejo Y, Tunold R (2004) J Appl Electrochem 34:1271–1278
Massalski TB, Murray JL, Benett LH, Baker H (1990) Binary alloy phase diagrams, American Society for Metals
Castrillejo Y, Bermejo MR, Barrado E, Martínez AM, Arocas PD (2003) J Electroanal Chem 545:141–157
Cordoba G, Caravaca C (2004) J Electroanal Chem 572:145–151
Castrillejo Y, de la Fuente C, Vega M, de la Rosa F, Pardo R, Barrado E (2013) Electrochim Acta 97:120–131
Plambeck JA (1976) Encyclopedia of Chemistry of the Elements. Marcel Dekker, New York
Bychkov AV, Skiba OV (2000) Pyrochemical separations: Workshop Proceeding OECD/NEA. Avignon, France
Chamelot P, Lafage B, Taxil P (1997) Electrochim Acta 43:607–616
Embarek B, Gilles MB, Jean M, François V, Mustayeen AK (2004) CR Chim 7:537–545
Chamelot P, Massot L, Hamel C, Nourry C, Taxil P (2007) J Nucl Mater 360:64–74
Acknowledgments
The work was financially supported by Key Laboratory of Superlight Materials and Surface Technology, Ministry of Education, the National 863 Project of Ministry of Science and Technology of China (no. 2011AA03A409), the Major Research plan of the National Natural Science Foundation of China (91226201 and 91326113), the National Natural Science Foundation of China (21173060 and 21271054), and the Fundamental Research funds for the Central Universities (HEUCF201310012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Zhang, M., Han, W. et al. Selective extraction of gadolinium from Sm2O3 and Gd2O3 mixtures in a single step assisted by MgCl2 in LiCl–KCl melts. J Solid State Electrochem 18, 843–850 (2014). https://doi.org/10.1007/s10008-013-2333-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2333-7