Abstract
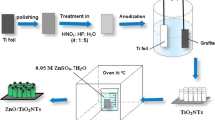
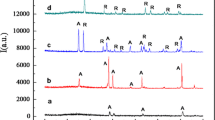
The photocatalytic conversion of CO2 and H2O to alcohols was achieved using self-organized TiO2 nanotube arrays (TNAs), which were prepared by electrochemical anodization of Ti foils in 1 M (NH4)2SO4 electrolyte containing 0.5 wt% NH4F. Experimental results revealed that the morphology and structure of self-organized TNAs could be strongly influenced by the applied voltage and anodization temperature, and the optimized TNAs were prepared by electrochemical anodization of Ti foils under optimal conditions (i.e., at 20 V for 2 h at 30 °C). The as-prepared TNAs were amorphous and could be transformed to anatase phase during the thermal treatment at 450 °C in air for 3 h. By using the annealed TNAs as a photocatalyst, the photocatalytic reduction of CO2 to alcohol, predominately methanol and ethanol, was demonstrated under Xenon lamp illumination. Based on the photocatalytic measurements, the production rates of methanol and ethanol were calculated to be ∼10 and ∼9 nmol cm−2 h−1, respectively. In addition, the formation mechanism of methanol and ethanol was also tentatively proposed.







Similar content being viewed by others
References
Lagowski JJ (1973) Modern inorganic chemistry. Marcel Dekker, New York
Yamashita H, Fujii Y, Ichihashi Y, Zhang SG, Ikeue K, Park DR, Koyano K, Tatsumi T, Anpo M (1998) Catal Today 45:221–227
Inoue T, Fujishima A, Konishi S, Honda K (1979) Nature 277:637–638
Thampi KR, Kiwi J, Grätzel M (1987) Nature 327:506–508
Cook RL, MacDuff RC, Sammells AF (1988) J Electrochem Soc 135:3069–3070
Anpo M, Yamashita H, Ichihashi Y, Ehara S (1995) J Electroanal Chem 396:21–26
Tseng IH, Wu JCS, Chou HY (2004) J Catal 221:432–440
Roy SC, Varghese OK, Paulose M, Grimes CA (2010) ACS Nanosci 4:1259–1278
Linsebigler AL, Lu GQ, Yates JT Jr (1995) Chem Rev 95:735–758
Fujishima A, Rao TN, Tryk DA (2000) J Photochem Photobiol C 1:1–21
Li JH, Zhang JZ (2009) Coord Chem Rev 253:3015–3041
Wang C, Thompson RL, Baltrus J, Matranga C (2010) J Phys Chem Lett 1:48–53
Ishitani O, Inoue C, Suzuki Y, Ibusuki T (1993) J Photochem Photobiol A 72:269–271
Adachi K, Ohta K, Mijuma T (1994) Sol Energy 53:187–190
Anpo M, Yamashita H, Ichihashi Y, Fujii Y, Honda M (1997) J Phys Chem B 101:2632–2636
Dimitrijevic NM, Vijayan BK, Poluektov OG, Rajh T, Gray KA, He H, Zapol P (2011) J Am Chem Soc 133:3964–3971
Wu CS, Wu TH, Chu T, Huang H, Tsai D (2008) Top Catal 47:131–136
Xia XH, Jia ZJ, Yu Y, Liang Y, Wang Z, Ma LL (2007) Carbon 45:717–721
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Chem Rev 95:69–96
Xi GC, Ouyang SX, Ye JH (2011) Chem Eur J 17:9057–9061
Varghese OK, Paulose M, Grimes CA (2009) Nat Nanotechnol 4:592–597
Wang G, Wang Q, Lu W, Li JH (2006) J Phys Chem B 110:22029–22034
Han XG, Kuang Q, Jin MS, Xie ZX, Zheng LS (2009) J Am Chem Soc 131:3152–3153
Li HX, Bian ZF, Zhu J, Zhang DQ, Li GS, Huo YN, Li H, Lu YF (2007) J Am Chem Soc 129:8406–8407
Gong D, Grimes CA, Varghese OK, Hu W, Singh RS, Chen Z, Dickey EC (2001) J Mater Res 16:3331–3334
Macak JM, Tsuchiya H, Ghicov A, Yasuda K, Hahn R, Bauer S, Schmuki P (2007) Curr Opin Solid State Mater Sci 11:3–18
Shankar K, Bandara J, Paulose M, Wietasch H, Varghese OK, Mor GK, LaTempa TJ, Grimes CA (2008) Nano Lett 8:1654–1659
Paulose M, Mor GK, Varghese OK, Shankar K, Grimes CA (2006) J Photochem Photobiol A 178:8–15
Koci K, Mateju K, Obalova L, Krejcikova S, Lacny Z, Placha D, Capek L, Hospodkova A, Solcova O (2010) Appl Catal, B 96:239–244
Macak JM, Zlamal M, Krysa J, Schmuki P (2007) Small 3:300–304
Mun KS, Alvarez SD, Choi WY, Sailor MJ (2010) ACS Nano 4:2070–2076
Zhao RR, Xu MZ, Wang J, Chen GN (2010) Electrochim Acta 55:5647–5651
Chen D, Zhang H, Li X, Li JH (2010) Anal Chem 82:2253–2261
Varghese K, Paulose M, LaTempa TJ, Grimes CA (2009) Nano Lett 9:731–737
Raja KS, Smith YR, Kondamudi N, Manivannan A, Misra M, Subramanian V (2011) Electrochem Solid-State Lett 14:F5–F8
Feng XJ, Sloppy JD, LaTempa TJ, Paulose M, Komarneni S, Bao NZ, Grimes CA (2011) J Mater Chem 21:13429–13433
Cao G, Rabenberg LK, Nunn CM, Mallouk TE (1991) Chem Mater 3:149–156
Hussain ST, Siddiqa A (2011) Int J Environ Sci Technol 8:351–362
Egerton TA, Tooley IR (2004) J Phys Chem B 108:5066–5072
Shimizu K, Tsuji Y, Hatamachi T, Toda K, Kodama T, Sato M, Kitayama Y (2004) Phys Chem Chem Phys 6:1064–1069
Li XK, Pan HQ, Li W, Zhuang ZJ (2012) Appl Catal A 413–414:103–108
Ishibashi K, Fijishima A, Watanabe T, Hashimoto K (2000) J Phys Chem B 104:4934–4938
Lo CC, Hung CH, Yuan CS, Hung YL (2007) Chin J Catal 28:528–534
Zhang QH, Han WD, Hong YJ, Yu JG (2009) Catal Today 148:335–340
Centi G, Perathoner S, Wine G, Gangeria M (2007) Green Chem 9:671–678
Takeda H, Ishitani O (2010) Coord Chem Rev 254:346–354
Indrakanti VP, Schobert HH, Kubicki JD (2009) Energy Fuel 23:5247–5256
Koci K, Obalova L, Matejova L, Placha D, Lacny Z, Jirkovsky J, Solcova O (2009) Appl Catal, B 89:494–502
Tseng IH, Chang WC, Wu JCS (2002) Appl Catal, B 37:37–48
Asi MA, He C, Su MH, Xia DH, Lin L, Deng HQ, Xiong Y, Qiu RL, Li XZ (2011) Catal Today 175:256–263
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (nos. 51072189, 21003111, and 11075147).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 5548 kb)
Rights and permissions
About this article
Cite this article
Ping, G., Wang, C., Chen, D. et al. Fabrication of self-organized TiO2 nanotube arrays for photocatalytic reduction of CO2 . J Solid State Electrochem 17, 2503–2510 (2013). https://doi.org/10.1007/s10008-013-2143-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2143-y