Abstract
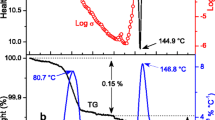
We report on the crystal structures of two hydrates of benzenehexasulfonic acid, its water sorption isotherm, temperature- and humidity-dependent conductivity, along with 1H NMR studies. At comparable humidities and temperatures, this crystalline material shows conductivity similar to Nafion, which conducts protons via liquid water channels. We believe that the presented discovery of fast protonic conductivity in benzenehexasulfonic acid at low humidities is encouraging for further efforts in developing highly sulfonated polymers as membranes for fuel cells.











Similar content being viewed by others
References
Gasteiger HA, Mathias MF (2002) Fundamental research and development challenges in polymer electrolyte fuel cell technology. Proton conducting membrane fuel cells III symposium. Electrochemical Society, Salt Lake City
Savadogo O (2004) Emerging membranes for electrochemical systems—part II. High temperature composite membranes for polymer electrolyte fuel cell (PEFC) applications. J Power Sources 127:135–161
Hogarth WHJ, da Costa JCD, Lu GQ (2005) Solid acid membranes for high temperature (>140 °C) proton exchange membrane fuel cells. J Power Sources 142:223–237
Kreuer KD (2000) On the complexity of proton conduction phenomena. Solid State Ion 136:149–160
Schuster M, Rager T, Noda A, Kreuer KD, Maier J (2005) About the choice of the protogenic group in PEM separator materials for intermediate temperature, low humidity operation: a critical comparison of sulfonic acid, phosphonic acid and imidazole functionalized model compounds. Fuel Cells 5:355–365
Doyle M, Rajendran G (2003) Perfluorinated membranes. In: Vielstich W, Lamm A, Gasteiger H (eds) Handbook of fuel cells: fundamentals, technology and applications. Wiley, New York, pp 352–395
Khiterer M, Loy DA, Cornelius CJ, Fujimoto CH, Small JH, McIntire TM, Shea KJ (2006) Hybrid polyelectrolyte materials for fuel cell applications: design, synthesis, and evaluation of proton-conducting bridged polysilsesquioxanes. Chem Mater 18:3665–3673
Rikukawa M, Sanui K (2000) Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog Polym Sci 25:1463–1502
Alberti G, Casciola M, Massinelli L, Bauer B (2001) Polymeric proton conducting membranes for medium temperature fuel cells (110–160°C). J Membr Sci 185:73–81
Miyatake K, Shouji E, Yamamoto K, Tsuchida E (1997) Synthesis and proton conductivity of highly sulfonated poly(thiophenylene). Macromolecules 30:2941–2946
Granados-Focil S, Litt MH (2004) New class of polyelectrolytes, polyphenylene sulfonic acids and its copolymers, as proton exchange membranes for PEMFC's. Abstracts of Papers of the American Chemical Society 228:U657–U657
Kang J (2008) A new class of polyelectrolyte; poly(p-phenylene disulfonic acids) macromolecular science and engineering dissertation case. Western Reserve University, Cleveland, OH, p 313
Taninouchi Y, Hatada N, Uda T, Awakura Y (2009) Phase relationship of CsH2PO4–CsPO3 system and electrical properties of CsPO3. J Electrochem Soc 156:B572–B579
Dokunikhin NS, Gaeva LA, Mezentseva GA (1972) Benzenehexasulfonic acid. Dokl Akad Nauk SSSR 206:624–626
Dokunikhin NS, Mezentseva GA (1976) Higher polysulfonic acids of benzene. Zhurnal Organicheskoi Khimii 12:621–625
Khmelnitskaya EY, Mezentseva GA, Dokunikhin NS (1980) Electrochemical reduction of benzene hexasulfonic, pentasulfonic, and tetrasulfonic acids. Sov Electrochem 16:940–945
Maksimov YM, Cheshchevoi VN, Podlovchenko BI (1985) Effect of the structures of aromatic sulfonic acids on their adsorption at platinum electrodes. Vestnik Moskovskogo Universiteta, Seriya 2: Khimiya 26:477–479
Chetkina LA, Sobolev AN, Mezentseva GA, Dokunikhin NS (1975) Structure of crystals of octahydrate of hexasodium salt of benzenehexasulfonic acid. Doklady AN SSSR 220:1343–1346
Chetkina LA, Sobolev AN (1977) The crystal structure of the hexasodium salt of benzenehaxasulfonic acid octahydrate. Acta Cryst B 33:2751–2756
Berckmans VSF, Holleman AF (1925) The tetrachloronitrobenzenes, the tetrachlorodinitrobenzenes; their reaction with sodium methylate. Anales Soc Espan Fis Quim 23:358–371
Garanin EM, Tolmachev YV (2008) Apparatus for measurement of protonic conductivity of powdered materials as a function of temperature and humidity. J Electrochem Soc 155:B1251–B1254
March J (1992) Advanced organic chemistry, 4th edn. Wiley, New York
Garanin EM, Tolmachev YV, Hoover RR, Adas S, Bunge SD, Gangoda M, Khitrin AK, Woods S, Malkovskiy A, Solak N, Wesdemiotis C (2010) Stability and tautomerization of cyclic anhydrides of benzenehexasulfonic acid. J Org Chem (in press)
Collard DM, Sadri MJ, Vanderveer D, Hagen KS (1995) Highly twisted substituted Arenes—X-ray structure and dynamic H-NMR spectra of 1, 4-dialkyl-2, 3, 5, 6-tetrakis(alkylsulfonyl)benzenes. J Chem Soc Chem Commun 1995:1357–1358
Attig R (1976) Cryst Struct Commun 5:223
Attig R, Mootz D (1976) Acta Cryst B 32:435
Lundgren JO (1972) Acta Cryst B 28:1684
Skakle JMS, Wardell JL (2006) Acta Cryst E 62:o1402
Devlin JP, Severson MW, Mohamed F, Sadlej J, Buch V, Parrinello M (2005) Experimental and computational study of isotopic effects within the Zundel ion. Chem Phys Lett 408:439–444
Koleva V, Stefov V, Cahil A, Najdoski M, Soptrajanov B, Engelen B, Lutz HD (2009) Infrared and Raman studies of manganese dihydrogen phosphate dihydrate, Mn(H2PO4)2x2H2O. Part II: region of the internal OH group vibrations. J Mol Struct 919:164–169.
Zarubin DP (1999) Infrared spectra of hydrogen bonded hydroxyl groups in silicate glasses. A re-interpretation. Phys Chem Glasses 40:184–192
Baumer U, Boldt K, Engelen B, Muller H, Unterderweide K (1999) Preparation, crystal structure and IR spectra of BeSeO3 x H2O–hydrogen bonds and correlation of IR and structure data in the monohydrates MSeO3 x H2O (M=Be, Ca, Mn, Co, Ni, Zn, Cd). Zeitschrift Fur Anorganische Und Allgemeine Chemie 625:395–401
Choi BK, Lee MN, Kim JJ (1989) Raman-spectra of the NaH2PO4 crystal. J Raman Spectrosc 20:11–15
Colomban P (1992) Proton conductors: solids, membranes and gels—materials and devices. Chemistry of solid state materials. Cambridge University Press, Cambridge, p 581
Lutz HD (2003) Structure and strength of hydrogen bonds in inorganic solids. J Mol Struct 646:227–236
Mikenda W (1986) Stretching frequency versus bond distance correlation of O–D(H)...Y (Y=N, O, S, Se, Cl, Br, I) hydrogen bonds in solid hydrates. J Mol Struct 147:1–15
Novak A (1974) Hydrogen bonding in solids. Correlation of spectroscopic and crystallographic data. In: Dunitz JD (ed) Structure and bonding. Springer, Berlin, pp 117–216
Unterderweide K, Engelen B, Boldt K (1994) Strong hydrogen-bonds in acid selenites—correlation of infrared spectroscopic and structural data. J Mol Struct 322:233–239
Hermansson K, Gajewski G, Mitev PD (2008) Pressure-induced OH frequency downshift in Brucite: frequency–distance and frequency field correlations. J Phys Conf Ser 117:012018
Bratos S, Leicknam JC, Pommeret S (2009) Time-resolved infrared spectroscopy of water. Relation between the OH stretching frequency and the O-O distance. Pol J Chem 83:737–745
Bomann D, Tilloy S, Monflier E (1999) Comparative Raman spectroscopy study of sulfonate-substituted triphenylphosphines. Vibr Spectrosc 20:165–172
Baranov AI (2003) Crystals with disordered hydrogen-bond networks and superprotonic conductivity. Review. Crystallogr Rep 48:1012–1037
Moller H, Mullerwarmuth W, Ruschendorf F, Schollhorn R (1987) Zeitschrift Fur Physikalische Chemie Neue Folge 151:121–131
Garanin EM, Towers MS, Toothaker PW, Laali K, Tolmachev YV (2010) Conductivity of highly sulfonated polyphenylene sulfide in the powder form as a function of temperature and humidity. Polymer Bull 64:595–605
Acknowledgment
The funding for this work was provided by the US Department of Energy, US National Science Foundation, Ohio Department of Development, Farris Family Innovation Fund and Kent State University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM. 1
(PDF 121 kb)
Rights and permissions
About this article
Cite this article
Garanin, E.M., Tolmachev, Y.V., Bunge, S.D. et al. Fast protonic conductivity in crystalline benzenehexasulfonic acid hydrates. J Solid State Electrochem 15, 549–560 (2011). https://doi.org/10.1007/s10008-010-1094-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-010-1094-9