Abstract
Context
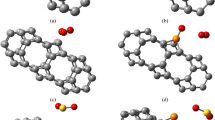
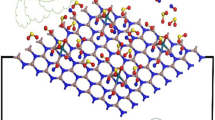
Langmuir adsorption of gas molecules of NO, NO2, and NH3 on the graphitic GaN and GaP sheets has been accomplished using density functional theory. The changes of charge density have shown a more important charge transfer for GaN compared to GaP which acts both as the electron donor while gas molecules act as the stronger electron acceptors through adsorption on the graphitic-like GaN surface. The adsorption of NO and NO2 molecules introduced spin polarization in the PL-GaN sheet, indicating that it can be employed as a magnetic gas sensor for NO and NO2 sensing.
Methods
The partial electron density states based on “PDOS” graphs have explained that the NO and NO2 states in both of GaN and GaP nanosheets, respectively, have more of the conduction band between − 5 and − 10 eV, while expanded contribution of phosphorus states is close to gallium states, but nitrogen and oxygen states have minor contributions. GaN and GaP nanosheets represent having enough capability for adsorbing gases of NO, NO2, and NH3 through charge transfer from nitrogen atom and oxygen atom to the gallium element owing to intra-atomic and interatomic interactions. Ga sites in GaN and GaP nanosheets have higher interaction energy from Van der Waals’ forces with gas molecules.







Similar content being viewed by others
Data availability
It is not applicable.
References
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: Buckminsterfullerene. Nature 318:162–163
Nasibulin AG, Pikhitsa PV, Jiang H, Brown DP, Krasheninnikov AV, Anisimov AS, Queipo P, Moisala A, Gonzalez D, Lientschnig G et al (2007) A Novel Hybrid Carbon Material. Nat Nanotechnol 2:156–161
Moisala A, Nasibulin AG, Shandakov SD, Jiang H, Kauppinen EI (2005) On-Line Detection of single-walled carbon nanotube formation during aerosol synthesis methods. Carbon 43:2066–2074
Mollaamin F, Monajjemi M (2023) Doping of graphene nanostructure with iron, nickel and zinc as selective detector for the toxic gas removal: a density functional theory study. C 9:20. https://doi.org/10.3390/c9010020.
Schedin F, Geim AK, Morozov SV, Hill EW, Blake P, Katsnelson MI, Novoselov KS (2007) Nat Mater 6:652–655
He Q, Wu S, Yina Z, Zhang H (2012) Chem Sci 3:1764–1772
Monajjemi M, Khaleghian M, Tadayonpour N, Mollaamin F (2010) The effect of different solvents and temperatures on stability of single-walled carbon nanotube: a QM/MD study. Int J Nanosci 09:517–529. https://doi.org/10.1142/S0219581X10007071
Mollaamin F, Ilkhani A, Sakhaei N, Bonsakhteh B, Faridchehr A, Tohidi S, Monajjemi M (2015) Thermodynamic and solvent effect on dynamic structures of nano bilayer-cell membrane: hydrogen bonding study. J Comput Theor Nanosci 12:3148–3154. https://doi.org/10.1166/jctn.2015.4092
Khalili Hadad B, Mollaamin F, Monajjemi M (2011) Biophysical chemistry of macrocycles for drug delivery: a theoretical study. Russ Chem Bull 60:238–241. https://doi.org/10.1007/s11172-011-0039-5
Su Y, Wang J, Wang B, Yang T, Yang B, Xie G, Zhou Y, Zhang S, Tai H, Cai Z et al (2020) Alveolus-inspired active membrane sensors for self-powered wearable chemical sensing and breath analysis. ACS Nano 14:6067–6075. https://doi.org/10.1021/acsnano.0c01804
Ma D, Zhang J, Li X, He C, Lu Z, Lu Z, Lu Z, Yang Z, Wang Y (2018) C3N monolayers as promising candidates for NO2 sensors. Sens Actuators B Chem 266:664–673. https://doi.org/10.1016/j.snb.2018.03.159
Mollaamin F (2014) On the behavior of boron nitride nanotube-flavin adenine dinucleotide interaction ion implantation order to design biofuel cells. J Comput Theor Nanosci 11(9):2017–2022
Tahan A, Mollaamin F, Monajjemi M (2009) Thermochemistry and NBO analysis of peptide bond: investigation of basis sets and binding energy. Russ J Phys Chem A 83:587–597. https://doi.org/10.1134/S003602440904013X
Monajjemi M, Baie MT, Mollaamin F (2010) Interaction between threonine and cadmium cation in [Cd(Thr)] (n = 1–3) complexes: density functional calculations. Russ Chem Bull 59:886–889. https://doi.org/10.1007/s11172-010-0181-5
Zadeh MA (2015) Akbari; Lari, Hadi; Kharghanian, Leyla; Balali, Ebrahim; Khadivi, Ramona; Yahyaei, Hooriye; Mollaamin, Fatemeh; Monajjemi, Majid, Density functional theory study and anti-cancer properties of shyshaq plant: in view point of nano biotechnology. J Comput Theor Nanosci 12:4358–4367. https://doi.org/10.1166/jctn.2015.4366
Mollaamin F, Monajjemi M, Salemi S, Baei MT (2011) A dielectric effect on normal mode analysis and symmetry of BNNT Nanotube. Fuller Nanotub Carbon Nanostructures 19:182–196. https://doi.org/10.1080/15363831003782932
Lee SW, Lee W, Hong Y, Lee G, Yoon DS (2018) Recent advances in carbon material-based NO2 gas sensors. Sens Actuators B Chem 255:1788–1804. https://doi.org/10.1016/j.snb.2017.08.203
Mollaamin F, Monajjemi M (2023) Corrosion inhibiting by some organic heterocyclic inhibitors through Langmuir adsorption mechanism on the Al-X (X = Mg/Ga/Si) alloy surface: a study of quantum three-layer method of CAM-DFT/ONIOM. J Bio Tribo Corros 9:33. https://doi.org/10.1007/s40735-023-00751-y
Xiao Z, Kong LB, Ruan S, Li X, Yu S, Li X, Jiang Y, Yao Z, Ye S, Wang C et al (2018) Recent development in nanocarbon materials for gas sensor applications. Sens Actuators B Chem 274:235–267. https://doi.org/10.1016/j.snb.2018.07.040
Khan MAH, Rao MV, Li Q (2019) Recent advances in electrochemical sensors for detecting toxic gases: NO2, SO2 and H2S. Sensors 19:905. https://doi.org/10.3390/s19040905
Bakhshi K, Mollaamin F, Monajjemi M (2011) Exchange and correlation effect of hydrogen chemisorption on nano V(100) surface: a DFT study by generalized gradient approximation (GGA). J Comput Theor Nanosci 8:763–768. https://doi.org/10.1166/jctn.2011.1750
Mahdavian L, Monajjemi M (2010) Alcohol sensors based on SWNT as chemical sensors: Monte Carlo and Langevin dynamics simulation. Microelectron J 41:142–149. https://doi.org/10.1016/j.mejo.2010.01.011
Johansson JKE, Mellqvist J, Samuelsson J, Offerle B, Lefer B, Rappenglück B, Flynn J, Yarwood G (2014) Emission measurements of alkenes, alkanes, SO2, and NO2 from stationary sources in Southeast Texas over a 5 year period using SOF and mobile DOAS. J Geophys Res Atmos 119:1973–1991. https://doi.org/10.1002/2013JD020485
Clark T, Murray JS, Politzer P (2018) A perspective on quantum mechanics and chemical concepts in describing noncovalent interactions. Phys Chem Chem Phys 20(48):30076–30082. https://doi.org/10.1039/C8CP06786D
Cui S, Pu H, Wells SA, Wen Z, Mao S, Chang J, Hersam MC, Chen J (2015) Nat Commun 6:8632
Batmunkh M, Bat-Erdene M, Shapter JG (2016) Adv Mater 28:8586–8617
Hussain T, Kaewmaraya T, Chakraborty S, Ahuja R (2016) J Phys Chem C 120:25256–25262
Chandramouli R, Srivastava A, Nagarjan V (2015) Appl Surf Sci 351:662–672
Duan X, Lieber CM (2000) General synthesis of compound semiconductor nanowires. Adv Mater 12:298–302
Owens A, Peacock A (2004) Compound semiconductor radiation detectors. Nucl Instrum Methods Phys Res Sect A Accel Spectrometers Detect Assoc Equip 531:18–37
Ikawa Y, Lee K, Ao J-P, Ohno Y (2014) Two-dimensional device simulation of AlGaN/GaN heterojunction FET side-gating effect. Jpn J Appl Phys 53:114302
Podolska A, Seeber RM, Mishra UK, Pfleger KDG, Parish G, Nener BD (2012) Detection of biological reactions by AlGaN/GaN biosensor. In Proceedings of the COMMAD 2012, Melbourne, Australia, 12–14 December 2012; pp. 75–76.
Prokopuk N, Son K-A, George T, Moon JS (2005) Development of GaN-based micro chemical sensor nodes. In Proceedings of the IEEE Sensors 2005, Irvine, CA, USA, 30 October–3 November 2005; pp. 199–202.
Pearton SJ, Kang BS, Kim SK, Ren FX, Gila B, Abernathy CR, Lin J, Chu G (2004) GaN-based diodes and transistors for chemical, gas, biological and pressure sensing. J Phys Condens Matter 16:R961–R994
Abdulsattar MA (2016) Superlattices Microstruct 93:163–170
Khan MS, Srivastava A (2016) J Electroanal Chem 775:243–250
Cui H, Yong Y, Jiang H, Yang L, Wang S, Zhang G, Guo M, Li X (2017) Mater Res Express 4:015009
Yong Y, Jiang H, Li X, Lv S, Cao J (2016) Phys Chem Chem Phys 18:21431–21441
Baik KH, Kim J, Jang S (2017) Sens Actuators B 238:462–467
Yong Y, Cui H, Zhou Q, Xiangying Su, Kuangb Y, Li X (2017) Adsorption of gas molecules on a graphitic GaN sheet and its implications for molecule sensors. RSC Adv 7:51027–51035. https://doi.org/10.1039/c7ra11106a
Khan MAH (2020) Gallium Nitride (GaN) Nanostructures and their gas sensing properties: a review. Sensors 20:3889. https://doi.org/10.3390/s20143889
Kente T, Mhlanga SD (2016) Gallium nitride nanostructures: synthesis, characterization and applications. J Cryst Growth 444:55–72
Mollaamin F, Monajjemi M (2023) In silico-DFT investigation of nanocluster alloys of Al-(Mg, Ge, Sn) coated by nitrogen heterocyclic carbenes as corrosion inhibitors. J Clust Sci. https://doi.org/10.1007/s10876-023-02436-5
Dural N, Romalis MV (2014) Gallium phosphide as a new material for anodically bonded atomic sensors. APL Mater 2(8). https://doi.org/10.1063/1.4891375.
Park K, Lee J, Kim D, Seo J, Kim J, Ahn J-P, Park J (2019) Synthesis of polytypic gallium phosphide and gallium arsenide nanowires and their application as photodetectors. ACS Omega 4(2):3098–3104. https://doi.org/10.1021/acsomega.8b03548
Šetka M, Claros M, Chmela O, Vallejos S (2021) Photoactivated materials and sensors for NO2 monitoring. J Mater Chem C 9:16804–16827. https://doi.org/10.1039/D1TC04247E
Geng X, Liu X, Mawella-Vithanage L, Hewa-Rahinduwage CC, Zhang L, Brock SL, Tan T, Luo L (2021) Photoexcited NO2 enables accelerated response and recovery kinetics in light-activated NO2 gas sensing. ACS Sens 6:4389–4397. https://doi.org/10.1021/acssensors.1c01694
Shin J, Han S, Noh S, Yu Y-T, Kim JS (2021) Room-temperature operation of light-assisted NO2 gas sensor based on GaN nanowires and graphene. Nanotechnology 32:505201. https://doi.org/10.1088/1361-6528/ac2427
Pinder RW, Walker JT, Bash JO, Cady-Pereira KE, Henze DK, Luo M et al (2011) Quantifying spatial and temporal variability in atmospheric ammonia with in situ and space-based observations. Geophys Res Lett 38(L04):802. https://doi.org/10.1029/2010GL046146
Langridge JM, Lack D, Brock CA, Bahreini R, Middlebrook AM, Neuman JA et al (2012) Evolution of aerosol properties impacting visibility and direct climate forcing in an ammonia-rich urban environment. J Geophys Res-Atmos 117:11. https://doi.org/10.1029/2011jd017116
Lamarque JF, Kyle G, Meinshausen M, Riahi K, Smith S, van Vuuren D et al (2011) Global and regional evolution of short-lived radiatively-active gases and aerosols in the representative concentration pathways. Clim Chang 109:191–212
Kondratev VM, Kuznetsov A, Fedina SV, Nalimova SS, Moshnikov VA, Bolshakov AD (2022) Gallium phosphide nanowires for “biological concentrations” ammonia detection. J Phys Conf Ser 2172:012006. https://doi.org/10.1088/1742-6596/2172/1/012006
Monajjemi M, Mahdavian L, Mollaamin F, Khaleghian M (2009) Interaction of Na, Mg, Al, Si with carbon nanotube (CNT): NMR and IR study. Russ J Inorg Chem 54:1465–1473. https://doi.org/10.1134/S0036023609090216
Mollaamin F, Monajjemi M (2023) Molecular modelling framework of metal-organic clusters for conserving surfaces: Langmuir sorption through the TD-DFT/ONIOM approach. Mol Simul 49(4):365–376. https://doi.org/10.1080/08927022.2022.2159996
Singh AK, Zhuang HL, Hennig RG (2014) Phys Rev B: Condens Matter Mater Phys 89:245431
Dennington R, Keith TA, Millam JM (2016) GaussView. Version 6. Shawnee Mission (KS): Semichem Inc.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H et al. (2016) Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865
Henkelman G, Arnaldsson A, Jónsson H (2006) A fast and robust algorithm for Bader decomposition of charge density. Comput Mater Sci 36(3):354–360
Zhou YG, Zu XT, Gao F et al (2009) Electronic and magnetic properties of graphene absorbed with S atom: a first-principles study. J Appl Phys 105(10):104311
Mao Y, Yuan J, Zhong J (2008) Density functional calculation of transition metal adatom adsorption on graphene. J Phys: Condens Matter 20(11):115209
Mollaamin F, Monajjemi M (2023) Graphene embedded with transition metals for capturing carbon dioxide: gas detection study using QM methods. Clean Technol 5(1):403–417. https://doi.org/10.3390/cleantechnol5010020
Mollaamin F, Shahriari S, Monajjemi M, Khalaj Z (2022) Nanocluster of aluminum lattice via organic inhibitors coating: a study of Freundlich adsorption. J Cluster Science: 1–16. https://doi.org/10.1007/s10876-022-02335-1.
Mollaamin F, Monajjemi M (2023) Transition metal (X = Mn, Fe Co, Ni, Cu, Zn)-doped graphene as gas sensor for CO2 and NO2 detection: a molecular modeling framework by DFT perspective. J Mol Model 29:119. https://doi.org/10.1007/s00894-023-05526-3
Monajjemi M, Mollaamin F, Gholami MR, Yoosbashizadeh H, Sadrnezhad SK, Passdar H (2003) Quantum chemical parameters of some organic corrosion inhibitors, Pyridine, 2-Picoline 4-Picoline and 2,4-Lutidine, adsorption at aluminum surface in hydrochloric and nitric acids and comparison between two acidic media. Main Group Met Chem 26:349–362. https://doi.org/10.1515/MGMC.2003.26.6.349
Svensson M, Humbel S, Froese RDJ, Matsubara T, Sieber S, Morokuma K (1996) ONIOM: A multilayered integrated MO + MM method for geometry optimizations and single point energy predictions. A test for diels−Alder reactions and Pt(P(t-Bu)3)2 + H2 oxidative addition. J Phys Chem 100(50):19357–19363. https://doi.org/10.1021/jp962071j
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393(1–3):51–57. https://doi.org/10.1016/j.cplett.2004.06.011
Lehtola S (2019) A review on non-relativistic fully numerical electronic structure calculations on atoms and diatomic molecules. Int J Quantum Chem 119(19):25968. https://doi.org/10.1002/qua.25968
Smith JAS (1971) Nuclear quadrupole resonance spectroscopy. J Chem Educ 48:39–41
Appendix K: Nuclear quadrupole resonance, by Allen N. Garroway, Naval Research Laboratory. In Jacqueline MacDonald, J. R. Lockwood: alternatives for landmine detection. Report MR-1608, Rand Corporation, 2003.
Poleshchuck OKh, Kalinna EL, Latosinska JN, Koput J (2001) J Mol Struct (THEOCHEM) 547:233–243
Young HA, Freedman RD (2012) Sears and Zemansky’s University Physics with Modern Physics, 13th edn. Addison-Wesley, Boston, p 754
Fry A, K., D. Kwon, K., Komarneni, S., D. Kubicki, J., T. Mueller, K. (2006) Solid-state NMR and computational chemistry study of mononucleotides adsorbed to alumina. Langmuir 22:9281–9286
Kohn W, Becke AD, Parr RG (1996) Density functional theory of electronic structure. J Phys Chem 100:12974–12980. https://doi.org/10.1021/jp960669l
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Politzer P, Abu-Awwad F (1998) A comparative analysis of Hartree-Fock and Kohn-Sham orbital energies. Theor Chem Acc 99:83–87. https://doi.org/10.1007/s002140050307
Silverstein RM, Bassler GC, Morrill TC (1981) Spectrometric Identification of Organic Compounds, 5th edn. Wiley, New York
Acknowledgements
In successfully completing this paper and its research, the authors are grateful to Kastamonu University for their support through the office, library, and scientific websites.
Author information
Authors and Affiliations
Contributions
Fatemeh Mollaamin: conceptualization and idea, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft preparation, visualization, supervision, project administration.
Majid Monajjemi: methodology, software, formal analysis, investigation, data curation, writing—review and editing, visualization, resources.
Corresponding author
Ethics declarations
Ethical approval
The authors consent to participate and publish the data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mollaamin, F., Monajjemi, M. Tailoring and functionalizing the graphitic-like GaN and GaP nanostructures as selective sensors for NO, NO2, and NH3 adsorbing: a DFT study. J Mol Model 29, 170 (2023). https://doi.org/10.1007/s00894-023-05567-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05567-8