Abstract
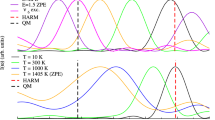
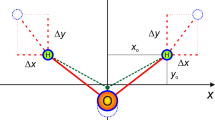
A comparison of four approaches to account the vibronic coupling in photoabsorption is performed. The methods considered are nuclear ensemble (NE), direct vibronic coupling (DVC), adiabatic Hessian (AH), and vertical gradient (VG). The case study is the symmetry-forbidden \(\tilde{X}\) \(^{1}\)A\(_1\) \(\rightarrow\) \(\tilde{A}\) \(^{1}\)A\(_2\) (n \(\rightarrow\) \(\pi ^*\)) transition in formaldehyde. Being forbidden in the equilibrium geometry, this transition is entirely induced by vibronic coupling and constitutes an appropriate case to study the performance of different methods. From DVC, it is found that mode 1 (C=O out-of-plane bending) is the most inducing, followed by mode 6 (in-plane C-H asymmetric stretching) and finally by mode 2 (in-plane C-H asymmetric bending). We were able to correlate 17 out of 20 structures obtained from NE with these modes, showing that these two methods, although different in principle, give comparable results. The simulated spectra were obtained for all methods and compared, and each one has its own advantage. In what concerns the transition studied, NE gives the best description of the spectrum, DVC is the only one that easily gives an absolute value for OOS, and AH and VG are the computationally less expensive methods. From the latter two, VG is the less demanding on computational grounds, since it does not require the excited state Hessian.






Similar content being viewed by others
Data availability
Data are contained in the main text and SI.
Code availability
Not applicable.
References
Condon EU (1947) The franck-condon principle and related topics. Am J Phys 15(5):365–374. https://doi.org/10.1119/1.1990977
Tapavicza E, Furche F, Sundholm D (2016) Importance of Vibronic Effects in the UV-Vis Spectrum of the 7,7,8,8-Tetracyanoquinodimethane Anion. J Chem Theory Comput 12(10):5058–5066. https://doi.org/10.1021/acs.jctc.6b00720
Zobel JP, Heindl M, Plasser F et al (2021) Surface hopping dynamics on vibronic coupling models. Acc Chem Res 54(20):3760–3771. https://doi.org/10.1021/acs.accounts.1c00485
Zuehlsdorff TJ, Shedge SV, Lu SY et al (2021) Vibronic and environmental effects in simulations of optical spectroscopy. Annu Rev Phys Chem 72(1):165–188. https://doi.org/10.1146/annurev-physchem-090419-051350
Ferrer FJ, Cerezo J, Stendardo E et al (2013) Insights for an accurate comparison of computational data to experimental absorption and emission spectra: Beyond the vertical transition approximation. J Chem Theory Comput 9(4):2072–2082. https://doi.org/10.1021/ct301107m
Zuehlsdorff TJ, Montoya-Castillo A, Napoli JA, et al (2019) Optical spectra in the condensed phase: Capturing anharmonic and vibronic features using dynamic and static approaches. J Chem Phys 151(7). https://doi.org/10.1063/1.5114818
Popp W, Polkehn M, Hughes KH, et al (2019) Vibronic coupling models for donor-acceptor aggregates using an effective-mode scheme: Application to mixed Frenkel and charge-transfer excitons in oligothiophene aggregates. J Chem Phys 150(24). https://doi.org/10.1063/1.5100529
Spano FC, Silva C (2014) H- and J-aggregate behavior in polymeric semiconductors. Annu Rev Phys Chem 65:477–500. https://doi.org/10.1146/annurev-physchem-040513-103639
Barford W, Marcus M (2017) Perspective: Optical spectroscopy in π-conjugated polymers and how it can be used to determine multiscale polymer structures. J Chem Phys 146(13). https://doi.org/10.1063/1.4979495
De Souza B, Neese F, Izsák R (2018) On the theoretical prediction of fluorescence rates from first principles using the path integral approach. J Chem Phys 148(3). https://doi.org/10.1063/1.5010895
Barbara PF, Meyer TJ (1996) Contemporary issues in electron transfer research. J Phys Chem 100(13):148–168. https://doi.org/10.1021/jp9605663
Santoro F, Jacquemin D (2016) Going beyond the vertical approximation with time-dependent density functional theory. Wiley Interdiscip Rev: Comput Mol Sci 6(5):460–486. https://doi.org/10.1002/wcms.1260
Charaf-Eddin A, Planchat A, Mennucci B et al (2013) Choosing a functional for computing absorption and fluorescence band shapes with TD-DFT. J Chem Theory Comput 9(6):2749–2760. https://doi.org/10.1021/ct4000795
Fang C, Oruganti B, Durbeej B (2014) How method-dependent are calculated differences between vertical, adiabatic, and 0-0 Excitation Energies? J Phys Chem A 118(23):4157–4171. https://doi.org/10.1021/jp501974p
Petrenko T, Neese F (2007) Analysis and prediction of absorption band shapes, fluorescence band shapes, resonance Raman intensities, and excitation profiles using the time-dependent theory of electronic spectroscopy. J Chem Phys 127(16). https://doi.org/10.1063/1.2770706
Kemper MJ, Lemmens L, Buck HM (1981) A comparative study of theoretical methods for calculating forbidden transitions. Chem Phys 57(1–2):245–252. https://doi.org/10.1016/0301-0104(81)80038-9
Rocha AB, Bielschowsky CE (2000) Vibronic coupling for H2CO and CO2. Chem Phys 253(1):51–57. https://doi.org/10.1016/S0301-0104(99)00379-1
Borges I Jr, Rocha AB, Bielschowsky CE (2005) Theoretical investigations on valence vibronic transitions. Braz J Phys 35(4a):971–980. https://doi.org/10.1590/S0103-97332005000600011
Crespo-Otero R, Barbatti M (2012) Spectrum simulation and decomposition with nuclear ensemble: formal derivation and application to benzene, furan and 2-phenylfuran. Theoret Chem Acc 131(6):1237. https://doi.org/10.1007/s00214-012-1237-4
Barbatti M, Aquino AJA, Lischka H (2010) The UV absorption of nucleobases: semi-classical ab initio spectra simulations. Phys Chem Chem Phys 12(19):4959. https://doi.org/10.1039/b924956g
Santoro F, Lami A, Improta R, et al (2007b) Effective method to compute vibrationally resolved optical spectra of large molecules at finite temperature in the gas phase and in solution. J Chem Phys 126(18). https://doi.org/10.1063/1.2721539
Santoro F, Improta R, Lami A, et al (2007a) Effective method to compute Franck-Condon integrals for optical spectra of large molecules in solution. J Chem Phys 126(8). https://doi.org/10.1063/1.2437197
Santoro F, Lami A, Improta R, et al (2008) Effective method for the computation of optical spectra of large molecules at finite temperature including the Duschinsky and Herzberg-Teller effect: The Qx band of porphyrin as a case study. J Chem Phys 128(22). https://doi.org/10.1063/1.2929846
Barone V, Bloino J, Biczysko M et al (2009) Fully integrated approach to compute vibrationally resolved optical spectra: from small molecules to macrosystems. J Chem Theory Comput 5(3):540–554. https://doi.org/10.1021/ct8004744
Rocha AB, Bielschowsky CE (2001) Intensity of the n → π* symmetry-forbidden electronic transition in acetone by direct vibronic coupling mechanism. Chem Phys Lett 337(4–6):331–334. https://doi.org/10.1016/S0009-2614(01)00213-5
Rocha AB, Pimentel AS, Bielschowsky CE (2002) Direct investigation of the validity of vertical approximation in the calculation of transition moment matrix elements: n → π* transition in methyl formate. J Phys Chem A 106(1):181–183. https://doi.org/10.1021/jp012647r
Borges I, Varandas AJ, Rocha AB et al (2003) Forbidden transitions in benzene. J Mol Struct (Thoechem) 621(1–2):99–105. https://doi.org/10.1016/S0166-1280(02)00537-7
Borges I, Rocha AB, Martínez-Núñez E et al (2005) Theoretical investigations on the vibronic coupling between the electronic states S0 and S1 of formic acid including the photodissociation at 248 nm. Chem Phys Lett 407(1–3):166–170. https://doi.org/10.1016/j.cplett.2005.03.077
Rocha AB (2007) Intensity of d-d symmetry-forbidden electronic transition in Cr(CO) 6. J Phys Chem A 111(21):4711–4713. https://doi.org/10.1021/jp070334b
Gomes AH, Oliveira RR, Rocha AB et al (2015) Strong Selectivity in Symmetry forbidden vibronic transitions in Deep Core Ionic Photofragmentation of the SF6 molecule. Int J Mass Spectrom 388:9–16. https://doi.org/10.1016/j.ijms.2015.07.019
Uhl E, Rocha AB, Leitão AA et al (2009) Intensity of d-s symmetry-forbidden electronic transition for Cu+ impurity in sodium chloride. Chem Phys Lett 483(1–3):72–76. https://doi.org/10.1016/j.cplett.2009.10.042
Uhl E, Leitão AA, Rocha AB (2011) Transition energies and oscillator strength calculated for d-s symmetry-forbidden electronic transition for Cu+ impurities in sodium fluoride host lattice. Chem Phys 389(1–3):102–106. https://doi.org/10.1016/j.chemphys.2011.08.011
Oliveira AP, Jalbert G, Rocha AB (2019) Generalized oscillator strengths of carbon disulfide calculated by multireference configuration interaction. J Chem Phys 150(17). https://doi.org/10.1063/1.5090613
Barbatti M, Ruckenbauer M, Plasser F et al (2014) Newton-X: a surface-hopping program for nonadiabatic molecular dynamics. WIREs Comput Mol Sci 4(1):26–33. https://doi.org/10.1002/wcms.1158
Barbatti M, Sen K (2016) Effects of different initial condition samplings on photodynamics and spectrum of pyrrole. Int J Quantum Chem 116(10):762–771. https://doi.org/10.1002/qua.25049
Arbelo-González W, Crespo-Otero R, Barbatti M (2016) Steady and time-resolved photoelectron spectra based on nuclear ensembles. J Chem Theory Comput 12(10):5037–5049. https://doi.org/10.1021/acs.jctc.6b00704
Barbatti M (2011) The role of tautomers in the UV absorption of urocanic acid. Phys Chem Chem Phys 13(10):4686–4692. https://doi.org/10.1039/c0cp02142c
Crespo-Otero R, Barbatti M (2011) Cr(CO)6 photochemistry: Semi-classical study of UV absorption spectral intensities and dynamics of photodissociation. J Chem Phys 134(16). https://doi.org/10.1063/1.3582914
Rocco MLM, Häming M, Moura CEVd, et al (2018) High-Resolution NEXAFS Study of Condensed Polyacenes. J Phys Chem C 122(50):692–701. https://doi.org/10.1021/acs.jpcc.8b08945
Cardozo TM, Aquino AJ, Barbatti M et al (2015) Absorption and fluorescence spectra of poly(p -phenylenevinylene) (PPV) oligomers: An ab initio simulation. J Phys Chem A 119(9):1787–1795. https://doi.org/10.1021/jp508512s
Soler J, Sarkar R, Boggio-Pasqua M (2019) Theoretical rationalization of the dual photophysical behavior of C 60+. J Phys Chem A 123(9):1824–1829. https://doi.org/10.1021/acs.jpca.8b11761
Born M, Oppenheimer R (1927) On the quantum theory of molecules. Ann Phys 389(20):457–484
Baiardi A, Bloino J, Barone V (2013) General time dependent approach to vibronic spectroscopy including franck-condon, herzberg-teller, and duschinsky effects. J Chem Theory Comput 9(9):4097–4115. https://doi.org/10.1021/ct400450k
Rappoport D, Furche F (2010) Property-optimized gaussian basis sets for molecular response calculations. J Chem Phys 133(134):105. https://doi.org/10.1063/1.3484283
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for h to rn: Design and assessment of accuracy. Phys Chem Chem Phys 7:3297. https://doi.org/10.1039/b508541a
Feller D (1996) The role of databases in support of computational chemistry calculations. J Comput Chem 17:1571–1586. https://doi.org/10.1002/(SICI)1096-987X(199610)17:13<1571::AID-JCC9>3.0.CO;2-P
Pritchard BP, Altarawy D, Didier B et al (2019) A new basis set exchange: An open, up-to-date resource for the molecular sciences community. J Chem Inf Model 59:4814–4820. https://doi.org/10.1021/acs.jcim.9b00725
Frisch MJ, Trucks GW, Schlegel HB, et al (2016) Gaussian ~16 Revision C.01. Gaussian Inc. Wallingford CT
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev: Comput Mol Sci 2(1):73–78
Neese F (2017) Software update: the ORCA program system, version 4.0. Wiley Interdiscip Rev: Comput Mol Sci 8(1):e1327
Pople JA, Sidman JW (1957) Intensity of the symmetry-forbidden electronic absorption band of formaldehyde. J Chem Phys 27(6):1270–1277. https://doi.org/10.1063/1.1743989
Roche M, Jaffé HH (1974) A modification of the Herzberg-Teller expansion for vibronic coupling. J Chem Phys 60(4):1193–1196. https://doi.org/10.1063/1.1681181
Johnson WC (1975) Calculation of vibronic intensity of the formaldehyde 1A 2?1A1 transition. J Chem Phys 63(5):2144–2148. https://doi.org/10.1063/1.431593
Strickler SJ, Barnhart RJ (1982) Absolute vibronic intensities in the 1A2 ← 1A1 absorption spectrum of formaldehyde. J Phys Chem 86(4):448–455. https://doi.org/10.1021/j100393a007
Gratien A, Nilsson E, Doussin JF, et al (2007) UV and IR absorption cross-sections of HCHO, HCDO, and DCDO. J Phys Chem A 111(45):506–513. https://doi.org/10.1021/jp074288r
Max-Planck Institute for Chemistry (2007) Formaldehyde’s UV spectra. https://uvvis.mpchmainz.gwdg.de/uvvis/cross_sections/Organics%20(carbonyls)/Aldehydes(aliphatic)/CH2O_Gratien(2007)_298K_250-360nm.txt
Acknowledgments
The authors acknowledge Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) and Fundação de Amparo á Pesquisa do Estado do Rio de Janeiro (FAPERJ) for financial support. Also, the National Laboratory for Scientific Computing (LNCC/MCTI, Brazil) for providing HPC resources of the SDumont supercomputer, which have contributed to the research results reported within this paper (http://sdumont.lncc.br).
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) and Fundação de Amparo á Pesquisa do Estado do Rio de Janeiro (FAPERJ)
Author information
Authors and Affiliations
Contributions
Amanda D. Torres: computation, analysis and writing; Carlos E. V. de Moura: revision and writing; Ricardo R. Oliveira: advisory, revision and writing; Alexandre B. Rocha: conception, advisory, revision and writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: XXI - Brazilian Symposium of Theoretical Chemistry (SBQT2021)
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torres, A.D., de Moura, C.E.V., Oliveira, R.R. et al. Comparison among several vibronic coupling methods. J Mol Model 28, 253 (2022). https://doi.org/10.1007/s00894-022-05230-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05230-8