Abstract
A new approach to estimate atomic energies is introduced. The method is based on utilization of experimental ionization energies as well as conversion of “n-electron atomic systems” to n “one-electron systems.” Sample detail calculations are presented with typical graphs to show the distribution of different types of energy within an atom. The breakdown of atomic energies into kinetic, electron-nucleus attraction, and electron-electron repulsion is shown within an atom as well the total of energies of each type for elements. Then in a following step, the variations in kinetic, electron-electron, and electron-nucleus interaction energies of electrons as evidence for atomic shell changes are presented. Furthermore, the article overviews the spatial gaps between orbitals as an added evidence for existence of electronic shells. The findings in this article have significant implications for the structure of atoms and the layout of periodic table.

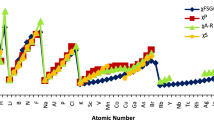
Electronic energy variations of barium (Ba) for kinetic, electron-electron repulsion, and electron-nucleus energies versus electron number












Similar content being viewed by others
References
Politzer P (1987) Single-particle density in physics and chemistry, chap. 3, edited by March NH, Deb BM, Academic, New York. ISBN 0-12-470518-9
Politzer P (2004). Theor Chem Accounts 111:395. https://doi.org/10.1007/s00214-003-0533-4
Politzer P, Murray JS (2002) The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor Chem Accounts 108:134–142. https://doi.org/10.1007/s00214-002-0363-9
Politzer P (2004) Some exact energy relationship. In: Brandas EJ, Kryachko ES (eds) Fundamental world of quantum chemistry, vol III. Kluwer Academic Publisher, Dordrecht, pp 631–638 ISBN: 1-4020-2583-1 (Vol III)
Politzer P (1980) Electrostatic potential–electronic density relationships in atoms. J Chem Phys 72:3027. https://doi.org/10.1063/1.439504
Politzer P (1981) Chemical applications of atomic and molecular electrostatic potentials. In: Politzer P, Truhlar DG (eds) pp 7–28. ISBN 978-1-4757-9634-6
Moller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618. https://doi.org/10.1103/PhysRev.46.618
Pople JA, Seeger R (1975) Electron density in Moller–Plesset theory. J Chem Phys 62:4566. https://doi.org/10.1063/1.430368
Levy M, Tal Y (1980) Atomic binding energies from fundamental theorems involving the electron density, <r−1>, and the Z−1 perturbation expansion. J Chem Phys 72:3416. https://doi.org/10.1063/1.439527
Levy M, Tal Y, Clement SC (1982) A discontinuous energy–density functional. J Chem Phys 77:3140. https://doi.org/10.1063/1.444237
Hellmann H (1937) Einfuhrung in die Quantenchemie. Deuticke, Leipzig. https://doi.org/10.1002/ange.19410541109
Feynman RP (1939) Forces in molecules. Phys Rev 56:340. https://doi.org/10.1103/PhysRev.56.340
Politzer P, Parr RG (1974) Some new energy formulas for atoms and molecules. J Chem Phys 61:4258. https://doi.org/10.1063/1.1681726
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev B 136:864. https://doi.org/10.1103/PhysRev.136.B864
March NH (1982) Electron density theory of atoms and molecules. J Phys Chem 86:2262. https://doi.org/10.1021/j100209a022
Milne EA (1927) The total energy of binding of a heavy atom. Proc Camb Philos Soc 23:794. https://doi.org/10.1017/S0305004100015589
Bohr N (1913) The spectra of helium and hydrogen. Nature 92:231–232. https://doi.org/10.1038/092231d0
Bohr N (1913) On the constitution of atoms and molecules, part I. Philos Mag 26(151):1–24. https://doi.org/10.1080/14786441308634955
Hehre WJ, Radom L, Schleyer PVR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York, pp 5–42. https://doi.org/10.1002/jcc.540070314
Sharpe AG (1986) Inorganic chemistry. Longman, London and New York, pp 32–61. https://doi.org/10.1002/bbpc.19860901144
Szabo A, Ostlund NS (1989) Modern quantum chemistry. McGraw-Hill, New York, pp 39–70 ISBN-13: 978-0486691862; ISBN-10: 0486691861
Murray JS, Zadeh DH, Lane P, Politzer P (2018) The role of ‘excluded’ electronic charge in noncovalent interactions. Mol Phys. https://www.tandfonline.com/doi/abs/10.1080/00268976.2018.1527044
Zadeh DH, Murray JS, Redfern PC, Politzer P (1991) Computational study of the nitrogen-nitro rotational energy barriers in some aliphatic and alicyclic nitramines. J Phys Chem 95(20):7702–7709. https://doi.org/10.1021/j100173a028
Zadeh DH, Grodzicki M, Seminario JM, Politzer P (1991) Computational study of the concerted gas-phase triple dissociations of 1, 3, 5-triazacyclohexane and its 1, 3, 5-trinitro derivative (RDX). J Phys Chem 95:7699. https://doi.org/10.1021/j100173a027
Zadeh DH, Murray JS, Grodzicki M, Seminario JM, Politzer P (1992) C-H bond dissociation of acetylene: local density functional calculations. Int J Quantum Chem 42:267–272. https://doi.org/10.1002/qua.560420203
Zadeh DH, Murray JS, Grice ME, Politzer P (1993) X–NO2 rotational energy barriers: local density functional calculations. Int J Quantum Chem 45:15–20. https://doi.org/10.1002/qua.560450104
Politzer P, Zadeh DH (1993) Relationship between dissociation energies, force constants, and bond lengths for some N–F and O–F bonds. J Chem Phys 98(9):7659. https://doi.org/10.1063/1.464679
Politzer P, Zadeh DH (1994) Bond-breaking energies for 2, 2′-dichlorodiethyl sulfide (sulfur mustard) in media of different dielectric constants. J Phys Chem 98:1576–1578. https://doi.org/10.1021/j100057a008
Grice ME, Zadeh DH, Politzer P (1994) Calculated structure, heat of formation and decomposition energetics of 1, 3-dinitro-1, 3-diazacyclobutane. J Chem Phys 100(6):4706–4707. https://doi.org/10.1063/1.466257
Zadeh DH, Grice ME, Concha MC, Murray JS, Politzer P (1995) Nonlocal density functional calculation of gas phase heats of formation. J Comput Chem 16(5):654–658. https://doi.org/10.1002/jcc.540160513
Politzer P, Concha MC, Grice ME, Murray JS, Lane P, Zadeh DH (1998) Computational investigation of the structures and relative stabilities of amino/nitro derivatives of ethylene. J Mol Struct (THEOCHEM) 452:75–83 https://homepage.univie.ac.at/mario.barbatti/papers/nitroethylene/politzer_theochem_1998.pdf
Moini S, Puri A, Zadeh DH, Das PC (1995) Ground state energy estimation of jellium systems by spatial gridding. Mod Phys Lett B 09:45. https://doi.org/10.1142/S0217984995000061
Orozco JGM, Luque FJ, Zadeh DH, Gao J (1995) The polarization contribution to the free energy of hydration. J Chem Phys 102:6145. https://doi.org/10.1063/1.469348
Gao J, Zadeh DH, Shao L (1995) A polarizable intermolecular potential function for simulation of liquid alcohols. J Phys Chem 99(44):16460–16467. https://doi.org/10.1021/j100044a039
Gao J, Pavelites JJ, Zadeh DH (1996) Simulation of liquid amides using a polarizable intermolecular potential function. J Phys Chem 100(7):2689–2697. https://doi.org/10.1021/jp9521969
Zadeh DH (2019) Atomic shells according to ionization energies. J Mol Model 25(8):251 https://link.springer.com/article/10.1007/s00894-019-4112-6
Zadeh DH (2017) Electronic structures of elements according to ionization energies. J Mol Model 23(12):357 https://link.springer.com/article/10.1007/s00894-017-3534-2
Kramida A, Yu R, Reader J, NIST ASD Team (2014) NIST Ionization Energy Database NIST Atomic Spectra Database (ver. 5.2). National Institute of Standards and Technology, Gaithersburg https://physics.nist.gov/asd. Accessed 9
Cotton FA, Wilkinson G (1989) Advanced inorganic chemistry5th edn. Wiley, New York. https://doi.org/10.1021/ed066pA104.2
McNaught AD, Wilkinson A (1997) IUPAC. Compendium of chemical terminology, the Gold Book2nd edn. Blackwell Science, Oxford ISBN 0865426848
Felker PM (2013) Fully quantal calculation of H2 translation-rotation states in (H2)4@51264 clathrate sII inclusion compounds. J Chem Phys 138:174306. https://doi.org/10.1063/1.4803117
Kollias AC, Domin D, Hill G, Frenklach M, Golden DM, Lester Jr WA (2005) Quantum Monte Carlo study of heats of formation and bond dissociation energies of small hydrocarbons, Int. Int J Chem Kinet 37:583. https://doi.org/10.1002/kin.20063
Aspuru-Guzik A, Salomon-Ferrer R, Austin B, Perusquia-Flores R, Griffin MA, Oliva RA, Skinner D, Domin D, Lester Jr WA (2005) Zori 1.0: a parallel quantum Monte Carlo electronic package. J Comput Chem 26:856. https://doi.org/10.1002/jcc.20215
Parol VJ, Sing-Long C, Adam Y, Bohm UL, Fan LZ, Farki SL, Cohen AE (2019) Compressed Hadamard microscopy for high-speed optically sectioned neuronal activity recordings. J Phys D: Appl Phys 52:144001. https://doi.org/10.1088/1361-6463/aafe88
Marsalek O, Markland TE (2016) Ab initio molecular dynamics with nuclear quantum effects at classical cost: ring polymer contraction for density functional theory. J. Chem. Phys 144(5):4112. https://doi.org/10.1063/1.4941093
Santra B, Klimes J, Tkatchenko A, Alfe D, Slater B, Michaelides A, Car R, Scheffler M (2013) On the accuracy of van der Waals inclusive density-functional theory exchange-correlation functionals for ice at ambient and high pressures. J Chem Phys 139(15):4702. https://doi.org/10.1063/1.4824481
Mauguiere FAL, Collins P, Stamatiadis S, Li A, Ezra GS, Farantos SC, Kramer ZC, Carpenter BK, Wiggins S, Guo H (2016) Toward understanding the roaming mechanism in H + MgH → Mg + HH reaction. J Phys Chem A 120(27):5145–5154. https://doi.org/10.1021/acs.jpca.6b00682
Sproviero EM, Gascón JA, McEvoy JP, Brudvig GW, Batista VS (2008) Quantum mechanics/molecular mechanics study of the catalytic cycle of water splitting in photosystem II. J Am Chem Soc 130(11):3428–3442. https://doi.org/10.1021/ja076130q
Liu R, Bloom BP, Waldeck DH, Zhang P, Beratan DN (2018) Improving solar cell performance using quantum dot triad charge-separation engines. J Phys Chem C 122(11):5924–5934. https://doi.org/10.1021/acs.jpcc.8b00010
Dunbar JA, Arthur EJ, White AM, Kubarych KJ (2015) Ultrafast 2D-IR and simulation investigations of preferential solvation and cosolvent exchange dynamics. J Phys Chem B 119(20):6271–6279. https://doi.org/10.1021/acs.jpcb.5b01952
Ding X, Vilseck JZ, Hayes RL, Brooks III CL (2017) Gibbs sampler-based lambda-dynamics and Rao-Blackwell estimator for alchemical free energy calculation. J Chem Theory Comput 13(6):2501–2510. https://doi.org/10.1021/acs.jctc.7b00204
Hoehn R, Carignano MA, Kais S, Francisco JS, Gladich I (2016) Hydrogen bonding and orientation effects on the accommodation of methylamine at the air-water interface. J Chem Phys 144:214701. https://doi.org/10.1063/1.4950951
Tiwary P, Berne BJ (2016) How wet should be the reaction coordinate in ligand unbinding. J Chem Phys 145:054113. https://doi.org/10.1063/1.4959969
Mondal J, Morrone J, Berne BJ (2013) How hydrophobic drying forces impact the kinetics of molecular recognition. Proc Natl Acad Sci U S A 110:13277–13282. https://doi.org/10.1073/pnas.1312529110
MacLeod MJ, Goodman AJ, Ye H-Z, Nguyen HV-T, Voorhis TV, Johnson JA (2018) Robust gold nanorods stabilized by bidentate N-heterocyclic-carbene–thiolate ligands. Nat Chem 11:57–63. https://doi.org/10.1038/s41557-018-0159-8
Grogan F, Holst M, Lindblom L, Amaro R (2017) Reliability assessment for large-scale molecular dynamics approximations. J Chem Phys 147(23):234106. https://doi.org/10.1063/1.5009431
Votapka LW, Jagger BR, Heyneman AL, Amaro RE (2017) SEEKR: simulation enabled estimation of kinetic rates, a computational tool to estimate molecular kinetics and its application to trypsin-benzamidine binding. J Phys Chem B 121(15):3597–3606. https://doi.org/10.1021/acs.jpcb.6b09388
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zadeh, D.H. A new approach to estimate atomic energies. J Mol Model 25, 366 (2019). https://doi.org/10.1007/s00894-019-4259-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4259-1