Abstract
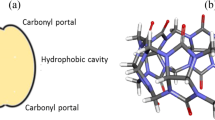
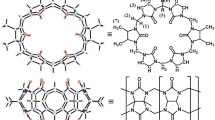
Understanding the interactions of cyclodextrins (CD) and cucurbit[n]uril (CB[n]) hosts with a variety of guest molecules following their encapsulation within the cavity of these macrocycles have become increasingly important in the recent years. The electronic charge distribution and the cavity dimension are some of the key factors those govern their interactions with cations or neutral guests. In the present work the molecular electrostatic potential (MESP) topography has been utilized to obtain the ‘effective’ cavity diameter and height of CB[n] (n = 6–8) homologues and 8 conformers each of α-, β- and γ-CD. It has been shown that the shape of CD cavity be it cone- or barrel-like stems from the hydrogen bonding patterns within primary hydroxyl groups. The width of CB[7] is comparable to the β-CD conformer that possesses either O6H–O5′ or intraglucose O6H–O5 interactions. The cavity diameters of α- and γ-CD are predicted to be respectively, 1.0 and 1.5 Å larger than CB[6] and CB[8] hosts. MESP topography reveals that the cavities of CB[n] are less hydrophilic with largely hydrophilic portals as compared to CD hosts. Cremer–Pople puckering parameters were derived for all the CD conformers and CB[n]. It has been demonstrated that the clockwise and anticlockwise hydrogen bonding patterns in the lower as well as upper rims of different CD conformers are less distorted and exhibit a little deviation from the °C3 chair conformation of α-d-glucopyranose constituting monomeric unit of CD.




Similar content being viewed by others
References
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998). doi:10.1021/cr970022c
Cunha-Silva, L., Teixeira-Dias, J.J.C.: How humidity affects the solid-state inclusion of 2-phenoxyethanol in β-cyclodextrin: a comparison with β-cyclodextrin. New J. Chem. 28, 200–206 (2004). doi:10.1039/b309491j
Vrielynck, L., Lapouge, C., Marquis, S., Kister, J., Dupuy, N.: Theoretical and experimental vibrational study of phenylurea: structure, solvent effect and inclusion process with the β-cyclodextrin in the solid state. Spectrochim. Acta A 60, 2553–2559 (2004). doi:10.1016/j.saa.2003.12.035
Al-Shihry, S.S.: Spectroscopic studies of inclusion complexes of 1-naphthol-4-sulfonate with β-cyclodextrin in aqueous solution. Spectrochim. Acta A 61, 2439–2443 (2005). doi:10.1016/j.saa.2004.09.006
Buchanan, C.M., Alderson, S.R., Cleven, C.D., Dixon, D.W., Ivanyi, R., Lambert, J.L., Lowman, D.W., Offerman, R.J., Szejtli, J., Szente, L.: Synthesis and characterization of water-soluble hydroxybutenyl cyclomaltooligosaccharides (cyclodextrins). Carbohydr. Res. 337, 493–507 (2002). doi:10.1016/S0008-6215(01)00328-7
Mosinger, J., Tomankova, V., Nemacova, I., Zyka, J.: Cyclodextrins in analytical chemistry. Anal. Lett. 34(12), 1979–2004 (2001). doi:10.1081/AL-100106834
Szejtli, J., Szente, L.: Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 61, 115–125 (2005). doi:10.1016/j.ejpb.2005.05.006
Redenti, E., Szente, L., Szejtli, J.: Cyclodextrin complexes of salts of acidic drugs. Thermodynamic properties, structural features, and pharmaceutical applications. J. Pharm. Sci. 90, 979–986 (2001). doi:10.1002/jps.1050
Redenti, E., Szente, L., Szejtli, J.: Drug/cyclodextrin/hydroxy acid multicomponent systems. Properties and pharmaceutical applications. J. Pharm. Sci. 89, 1–8 (2000). doi:10.1002/(SICI)1520-6017(200001)
Géczy, J., Bruhwyler, J., Scuvée-Moreau, J., Seutin, V., Masset, H., Van Heugen, J.C., Dresse, A., Lejeune, C., Decamp, E., Szente, L., Szejtli, J., Liégeois, J.-F.: The inclusion of fluoxetine into γ-cyclodextrin increases its bioavailability: behavioural, electrophysiological and pharmacokinetic studies. Psychopharmacology 151, 328–334 (2000). doi:10.1007/s002130000512
Loftsson, T., Jarvinen, T.: Cyclodextrins in ophthalmic drug delivery. Adv. Drug Deliv. Rev. 36, 59–79 (1999). doi:10.1016/S0169-409X(98)00055-6
Jeon, W.S., Moon, K., Park, S.H., Chun, H., Ko, Y.H., Lee, J.Y., Lee, E.S., Samal, S., Selvapalam, N., Rekharsky, M.V., Sindelar, V., Sobransingh, D., Inoue, Y., Kaifer, A.E., Kim, K.: Complexation of ferrocene derivatives by the cucurbit[7]uril host: a comparative study of the cucurbituril and cyclodextrin host families. J. Am. Chem. Soc. 127, 12984–12989 (2005). doi:10.1021/ja052912c
Buschmann, H.-J., Schollmeyer, E., Mutihac, L.: The formation of amino acid and dipeptide complexes with α-cyclodextrin and cucurbit[6]uril in aqueous solutions studied by titration calorimetry. Thermochim. Acta 399, 203–208 (2003). doi:10.1016/S0040-6031(02)00462-8
Mohanty, J., Bhasikuttan, A.C., Nau, W.M., Pal, H.: Host–guest complexation of neutral red with macrocyclic host molecules: contrasting pK a shifts and binding affinities for cucurbit[7]uril and β-cyclodextrin. J. Phys. Chem. B 110(10), 5132–5138 (2006). doi:10.1021/jp056411p
Moghaddam, S., Inoue, Y., Gilson, M.K.: Host–guest complexes with protein–ligand-like affinities: computational analysis and design. J. Am. Chem. Soc. 131(11), 4012–4021 (2009). doi:10.1021/ja808175m
Kim, J., Jung, I.-S., Kim, S.-Y., Lee, E., Kang, J.-K., Sakamoto, S., Yamaguchi, K., Kim, K.: New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7 and 8). J. Am. Chem. Soc. 122, 540–541 (2000). doi:10.1021/ja993376p
Koner, A.L., Nau, W.M.: Cucurbituril encapsulation of fluorescent dyes. Supramol. Chem. 19(1–2), 55–66 (2007). doi:10.1080/10610270600910749
Liu, J.-X., Long, L.-S., Huaang, R.-B.L., Zheng, L.-S.: Interesting anion-inclusion behavior of cucurbit[5]uril and its lanthanide-capped molecular capsule. Inorg. Chem. 46, 10168–10173 (2007). doi:10.1021/ic701236v
Osaka, I., Kondou, M., Selvapalam, N., Samal, S., Kim, K., Rekharsky, M.V., Inoue, Y., Arakawa, R.: Characterization of host–guest complexes of cucurbit[n]uril (n = 6, 7) by electrospray ionization mass spectrometry. J. Mass Spectrom. 41, 202–207 (2006). doi:10.1002/jms.978
Buschmann, H.-J., Cleve, E., Jansen, K., Wego, A., Schollmeyer, E.: Complex formation between cucurbit[n]urils and alkali, alkaline earth and ammonium ions in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 40, 117–120 (2001). doi:10.1023/A:1011159119554
Bhasikuttan, A.C., Mohanty, J., Nau, W.M., Pal, H.: Efficient fluorescence enhancement and cooperative binding of an organic dye in a supra-biomolecular host-protein assembly. Angew. Chem. Int. Ed. Engl. 46, 4120–4122 (2007). doi:10.1002/anie.200604757
Marquez, C., Hudgins, R.R., Nau, W.M.: Mechanism of host–guest complexation by cucurbituril. J. Am. Chem. Soc. 126(18), 5806–5816 (2004). doi:10.1021/ja0319846
Jeon, Y.J., Kim, S.-Y., Ko, Y.H., Sakamoto, S., Yamaguchi, K., Kim, K.: Novel molecular drug carrier: encapsulation of oxaliplatin in cucurbit[7]uril and its effects on stability and reactivity of the drug. Org. Biomol. Chem. 3, 2122–2125 (2005). doi:10.1039/b504487a
Kemp, S., Wheate, N.J., Stooman, F.H., Aldrich-Wright, J.R.: The host-guest chemistry of proflavine with cucurbit[6, 7, 8]urils. Supramol. Chem. 19, 475–484 (2007). doi:10.1080/10610270601124019
Kim, J., Ahn, Y., Park, K.M., Kim, Y., Ko, Y.H., Oh, D.H., Kim, K.: Carbohydrate wheels: cucurbituril-based carbohydrate clusters. Angew. Chem. Int. Ed. Engl. 119, 7537–7539 (2007). doi:10.1002/ange.200702540
Wei, F., Liu, S.-M., Xu, L., Cheng, G.-Z., Wu, C.-T., Feng, Y.-Q.: The formation of cucurbit[n]uril (n = 6, 7) complexes with amino compounds in aqueous formic acid studied by capillary electrophoresis. Electrophoresis 26, 2214–2224 (2005). doi:10.1002/elps.200410260
Tuncel, D., Steinke, J.H.G.: Catalytic self-threading: a new route for the synthesis of polyrotaxanes. Macromolecules 37, 288–302 (2004). doi:10.1021/ma034294v
Liu, S.-M., Wu, X., Huang, Z., Yao, J., Liang, F., Wu, C.: Construction of pseudorotaxanes and rotaxanes based on cucurbit[n]uril. J. Incl. Phenom. Macrocycl. Chem. 50, 203–207 (2004). doi:10.1007/s10847-004-6472-4
Corma, A., Garcıa, H., Montes-Navajas, P.A., Primo, J.J., Calvino, S., Trasobares, S.: Gold nanoparticles in organic capsules: a supramolecular assembly of gold nanoparticles and cucurbituril. Chemistry 13, 6359–6364 (2007). doi:10.1002/chem.200601900
Cervello, E., Jaime, C.: β-cyclodextrin bimodal complexes with n-alkylbenzenes and n-alkylcyclohexanes A molecular mechanics study. J. Mol. Struct. 428, 195–201 (1998). doi:10.1016/S0166-1280(97)00279-0
Madrid, J., Paozuelo, J., Mendicuti, F., Mattice, W.L.: Molecular mechanics study of the inclusion complexes of 2-methyl naphthoate with α- and β-cyclodextrins. J. Colloid Interface Sci. 193, 112–120 (1997). doi:10.1006/jcis.1997.5061
Margheritis, C., Sinistri, C.: β-cyclodextrin in aqueous solution: MM and semiempirical calculations. Farmaco 52, 435–438 (1997)
Casadesus, R., Moreno, M., Gonzalez-Lafont, A., Lluch, J.M., Repasky, M.P.: Testing electronic structure methods for describing intermolecular H···H interactions in supramolecular chemistry. J. Comput. Chem. 25, 99–105 (2004). doi:10.1002/jcc.10371
Britto, M.A.F.O., Nascimnento, C.S., Jr., Dos Santos, H.F.: Structural analysis of cyclodextrins: a comparative study of classical and quantum mechanical methods. Quim. Nova. 27(6), 882–888 (Portuguese) (2004); Sociedade Brasileira de Quimica (2004). doi:10.1590/S0100-40422004000600008
Nascimento Jr., C.S., Dos Santos, H.F., De Almedia, W.B.: Theoretical study of the formation of the α-cyclodextrin hexahydrate. Chem. Phys. Lett. 397, 422–428 (2004). doi:10.1016/j.cplett.2004.09.026
Nascimento Jr., C.S., Cleber, P.A., Dos Santos, H.F., De Almedia, W.B.: Theoretical study of the α-cyclodextrin dimer. J. Phys. Chem. A 109, 3209–3219 (2005). doi:10.1021/jp044490j
Bako, I., Jicsinszky, L.: Semiempirical calculations on cyclodextrins. J. Incl. Phenom. Mol. Recognit. Chem. 18(3), 275–289 (1994). doi:10.1007/BF00708734
Margheritis, C., Sinistri, C.: β-cyclodextrin and water semiempirical calculations. Z. Naturforsch. A 51(8), 950–956 (1996)
Avakyan, V.G., Nazarov, V.B., Alfimov, M.V., Bagatur’yants, A.A., Voronezheva, N.I.: The role of intra- and intermolecular hydrogen bonds in the formation of b-cyclodextrin head-to-head and head-to-tail dimers. The results of ab initio and semiempirical quantum- chemical calculations. Russ. Chem. Bull. 50(2), 206–216 (2001). doi:10.1023/A:1009557729668
Anconi, C.P.A., Nascimento Jr., C.S., Fedoce-Lopes, J., Dos Santos, H.F., De Almeida, W.B.: Ab initio calculations on low-energy conformers of α-cyclodextrin. J. Phys. Chem. A 111, 12127–12135 (2007). doi:10.1021/jp0762424
Karpfen, A., Liedl, E., Snor, W., Weiss-Greiler, P., Viernstein, H., Wolschann, P.: Homodromic hydrogen bonds in low-energy conformations of single molecule cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 57, 35–38 (2007). doi:10.1007/s10847-006-9166-2
Pinjari, R.V., Joshi, K.A., Gejji, S.P.: Molecular electrostatic potentials and hydrogen bonding in α-, β-, and γ-cyclodextrins. J. Phys. Chem. A 110, 13073–13080 (2006). doi:10.1021/jp065169z
Pinjari, R.V., Joshi, K.A., Gejji, S.P.: Theoretical studies on hydrogen bonding, NMR chemical shifts and electron density topography in α-, β-, and γ-cyclodextrins conformers. J. Phys. Chem. A 111, 13583–13589 (2007). doi:10.1021/jp074539w
El-Barghouthi, M.I., Jaime, C., Al-Sakhen, N.A., Issa, A.A., Adboth, A.A., Al Omari, M.M., Badwan, A.A., Zughul, M.B.: Molecular dynamics simulations and MM–PBSA calculations of the cyclodextrin inclusion complexes with 1-alkanols, para-substituted phenols and substituted imidazoles. J. Mol. Struct. 853, 45–52 (2008). doi:10.1016/j.theochem.2007.12.005
Pinjari, R.V., Gejji, S.P.: Electronic structure, molecular electrostatic potential, and NMR chemical shifts in cucurbit[n]urils (n = 5–8), ferrocene, and their complexes. J. Phys. Chem. A 112, 12679–12686 (2008). doi:10.1021/jp807268v
Carlqvist, P., Maseras, F.: A theoretical analysis of a classic example of supramolecular catalysis. Chem. Commun. 7, 748–750 (2007). doi:10.1039/b613434c
Buschmann, H.-J., Wego, A., Zielesny, A., Schollmeyer, E.: Structure, stability, electronic properties and NMR-shielding of the cucurbit[6]uril–spermine-complex. J. Incl. Phenom. Macrocycl. Chem. 54, 241–246 (2006). doi:10.1007/s10847-005-8140-8
Pichierri, F.: DFT study of cucurbit[n]uril, n = 5–10. J. Mol. Struct. 765, 151–152 (2006). doi:10.1016/j.theochem.2006.03.039
Oh, S.K., Yoon, J., Kim, K.S.: Structural stabilities and self-assembly of cucurbit[n]uril (n = 4–7) and decamethylcucurbit[n]uril (n = 4–6): a theoretical study. J. Phys. Chem. B 105, 9726–9731 (2001). doi:10.1021/jp011919n
Kaanumalle, L.S., Gibb, C.L.D., Gibb, B.C., Ramamurthy, V.: A hydrophobic nanocapsule controls the photophysics of aromatic molecules by suppressing their favored solution pathways. J. Am. Chem. Soc. 127, 3674–3675 (2005). doi:10.1021/ja0425381
Smit, B., Maesen, T.L.M.: Towards a molecular understanding of shape selectivity. Nature 451, 671–678 (2008). doi:10.1038/nature06552
Steed, J.W., Atwood, J.L. (eds.): Encyclopedia of Supramolecular Chemistry. Marcel Dekker, New York (2004)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchanges. J. Chem. Phys. 98, 5648–5652 (1993). doi:10.1063/1.464913
Lee, C., Yang, W., Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988). doi:10.1103/PhysRevB.37.785
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A. Jr., Vreven, T., Kudin, K.N., Burant. J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Aramillo, J.J., Gomperts, R., Stratmann, R.E., Yazyev O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S.J., Cioslowski, B.B., Stefanov, G., Liu, A., Liashenko, P., Piskorz, I., Komaromi, R.L., Martin, D.J., Fox, T.M.A., Keith, C.Y., Al-Laham, A., Peng, M., Nanayakkara, M., Challacombe, P.M.W., Gill, B., Johnson, W., Chen, M.W., Wong, C., Gonzalez, J. Pople, A.: Gaussian 03, Revision C.02. Gaussian, Wallingford (2004)
Balanarayan, P., Gadre, S.R.: Topography of molecular scalar fields. I. Algorithm and Poincaré–Hopf relation. J. Chem. Phys. 119, 5037–5043 (2003). doi:10.1063/1.1597652
Politzer, P., Trulhar, D.G. (eds.): Chemical Applications of Atomic and Molecular Electrostatic Potentials. Plenum, New York (1981)
Naray-Szabo, G., Ferenczy, G.G.: Molecular electrostatics. Chem. Rev. 4, 829–847 (1995). doi:10.1021/cr00036a002
Limaye, A.C., Gadre, S.R.: UNIVIS-2000: an indigenously developed comprehensive visualization package. Curr. Sci. 80, 1298–1301 (2001)
Cremer, D., Pople, J.A.: A general definition of ring puckering coordinates. J. Am. Chem. Soc. 97, 1354–1358 (1975). doi:10.1021/ja00839a011
Gessler, K., Krauss, N., Steiner, T., Betzel, C., Sarko, A., Saenger, W.: β-D-cellotetraose hemihydrate as a structural model for cellulose II. An X-ray diffraction study. J. Am. Chem. Soc. 117(46), 11397–11406 (1995). doi:10.1021/ja00151a003
Matta, C.F., Cow, C.N., Harrison, P.H.M.: Twisted amides: X-ray crystallographic and theoretical study of two acylated glycourils with aromatic substituents. J. Mol. Struct. 660, 81–97 (2003). doi:10.1016/j.molstruc.2003.08.005
Bekiroglu, S., Kenne, L., Sandstrom, C.: 1H NMR studies of maltose, maltoheptaose α-, β-, and γ-cyclodextrins, and complexes in aqueous solutions with hydroxy protons as structural probes. J. Org. Chem. 68, 1671 (2003). doi:10.1021/jo0262154
Brewster, M.E., Huang, M., Pop, E., Pitha, J., Dewar, M.J.S., Kaminski, J.J., Bodor, N.: An AM1 molecular orbital study of a-d-glucopyranose and β-maltose: evaluation and implications. Carbohydr. Res. 242, 53–67 (1993). doi:0008-6215/93/
Acknowledgements
SPG acknowledges support from the University Grants Commission (UGC), New Delhi, India [Research Project F34-370/2008(SR)] and University of Pune. RVP is grateful to Council of Scientific and Industrial Research, New Delhi, India for a Senior Research fellowship. JKK thanks UGC for the award of meritorious student fellowship. Authors thank the Center for Network Computing, University of Pune, for providing computational facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pinjari, R.V., Khedkar, J.K. & Gejji, S.P. Cavity diameter and height of cyclodextrins and cucurbit[n]urils from the molecular electrostatic potential topography. J Incl Phenom Macrocycl Chem 66, 371–380 (2010). https://doi.org/10.1007/s10847-009-9657-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9657-z