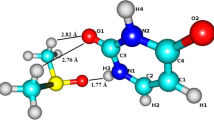
Abstract
The presented paper is focused on the calculation of hyperfine coupling constants (HFCC) of Cu 2+ ion in water environment. To simulate the conditions of the electron paramagnetic resonance (EPR) experiment in aqueous phase, molecular dynamics using the density functional theory (DFT) was employed. In total three different functionals (BLYP, B3LYP, M06) were employed for studying their suitability in describing coordination of Cu 2+ by water molecules. The system of our interest was composed of one Cu 2+ cation surrounded by a selected number (between thirty and fifty) of water molecules. Besides the non-relativistic HFCCs (Fermi contact terms) of Cu 2+ also the four-component relativistic HFCC calculations are presented. The importance of the proper evaluation of HFCCs, the inclusion of spin-orbit term, for Cu 2+ containing systems (Neese, J. Chem. Phys. 118, 3939 2003; Almeida et al., Chem. Phys. 332, 176 2007) is confirmed at the relativistic four-component level of theory.

Five and six coordinated copper dication is solvated by adding extra water molecules to simulate conditions in aqueous solution. Molecular dynamics study is performed and nonrelativistic and relativistic hyperfine coupling constants are calculated subsequently.







Similar content being viewed by others
References
Lippard SJ, Berg JM (1994) Principles of Bioinorganic Chemistry. University Science Books, Mill Valley
Bertini I, Gray HB, Stiefel EI, Valentine JS (2007) Biological Inorganic Chemistry. University Science Books, Mill Valley
Falconi M, Iacovelli F, Desideri A (2013) J Mol Model 19:3695
Boča R, Hvastijová M, Kožíšek J, Valko M (1996) Inorg Chem 35:4794
Jia LF, Fu WF, Yu MM, Cao QY, Zhang JF, Yin Q (2005) Inorg Chem Commun 8:647
Bérces A, Nukada T, Margl P, Ziegler T (1997) J Mol Struct 397:121
Stace AJ, Walker NR, Firth S (1997) J Am Chem Soc 119:10239
Allen FH (2002). Acta Cryst B 58:380
Conquest v1.17, csd v5.36 (nov 2014) Copyright CCDC 2014
Frank P, Benfatto M, Szilagyi RK, D’Angelo P, Longa SD, Hodgson KO (2005) Inorg Chem 44:1922
Benfatto M, D’Angelo P, Della Longa S, Pavel NV (2002) Phys Rev B 65:174205
Persson I, Persson P, Sandström M, Ullström AS (2002) J Chem Soc Dalton Trans 7:1256
Pasquarello A, Petri I, Salmon PS, Parisel O, Car R, Tóth E, Powell DH, Fischer HE, Helm L, Merbach AE (2001) Science 291:856
Chaboy J, Muñoz-Páez A, Merkling PJ, Marcos ES (2006) J Chem Phys 124:064509
Breza M, Biskupič S, Kožíšek J (1997) J Mol Struct 397:121
Car R, Parrinello M (1985) Phys Rev Lett 55:2471
Laasonen K, Pasquarello A, Car R, Lee C, Vanderbilt D (1993) Phys Rev B 47:10142
Amira S, Spȧngberg D, Hermansson K (2005) Phys Chem Chem Phys 7:2874
Texler NR, Rode BM (1995) J Phys Chem 99:15714
Marini GW, Liedl KR, Rode BM (1999) J Phys Chem A 103:11387
Schwenk CF, Rode BM (2003) J Chem Phys 119:9523
Schwenk CF, Rode BM (2003) Comp Phys Commun 4:931
Moin ST, Hofer TS, Weiss AKH, Rode BM (2013) J Chem Phys 139:014503
Blumberger J, Bernasconi L, Tavernelli I, Vuilleumier R, Sprik M (2004) J Am Chem Soc 126:3928
de Almeida KJ, Murugan NA, Rinkevicius Z, Hugosson HW, Vahtras O, Ågren H, Cesar A (2009) Phys Chem Chem Phys 11:508
de Almeida KJ, Rinkevicius Z, Hugosson HW, Ferreira AC, Ågren H (2007) Chem Phys 332:176
Lewis WB, Alei JM, Morgan LO (1966) J Chem Phys 44:2409
Rinkevicius Z, Telyatnyk L, Sałek P, Vahtras O, Ågren H (2004) J Chem Phys 121:7614
Neese F (2003) J Chem Phys 118:3939
Alder BJ, Wainwright TE (1957) J Chem Phys 27:1208
Kutzelnigg W (2002) Chapter 12. Perturbation theory of relativistic effects in Relativistic Electronic Structure Theory. Part I. Fundamentals. Elsevier, Amsterdam, p 664
Pyykkö P (1971) Phys Lett A 35:53
Komorovský S, Repiský M, Malkina OL, Malkin VG, Ondík IM, Kaupp M (2008) J Chem Phys 128:104101
Repiský M, Komorovský S, Malkin E, Malkina OL, Malkin VG (2010) Chem Phys Lett 488:94
Malkin E, Repiský M, Komorovský S, Mach P, Malkina OL, Malkin VG (2011) J Chem Phys 134:044111
Malkin V, Malkina O, Reviakine R, Arbuznikov A, Kaupp M, Schimmelpfennig B, Malkin I, Repiský M, Komorovský S, Hrobarik P, Malkin E, Helgaker T, Ruud K (2012) ReSpect Program. Version 3.2.0, 2012
Lee C, Yang W, Parr RG (1988). Phys Rev B 37:785
Becke AD (1988) Phys Rev A 38:3098
Becke AD (1993) J Chem Phys 98:5648
Vosko SH, Wilk L, Nusair M (1980) Can J Phys 58:1200
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623
Zhao Y, Truhlar DG (2006) J Chem Phys 125:194101
Hehre WJ, Ditchfield R, Pople JA (1972) J Chem Phys 56:2257
Rassolov VA, Pople JA, Ratner MA, Windus TL (1998) J Chem Phys 109:1223
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650
Wachters AJH (1970) J Chem Phys 52:1033
Dunning JTH (1989) J Chem Phys 90:1007
Balabanov NB, Peterson KA (2006) J Chem Phys 125:074110
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55:117
Barone V, Cossi M, Tomassi J (1997) J Chem Phys 107:3210
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian.09 Revision D.01. Gaussian Inc., Wallingford CT
Berendsen HJC, van der Spoel D, van Drunen R (1995) Comp Phys Commun 91:43
Valiev M, Bylaska EJ, Govind N, Kowalski K, Straatsma TP, van Dam HJJ, Wang D, Nieplocha J, Apra E, Windus TL, de Jong WA (2010) Comp Phys Commun 181:1477
Berendsen HJC, Postma JPM, van Gunsteren WF, Nola AD, Haak JR (1984) J Chem Phys 81:3684
Klamt A, Schüürmann G (1993) J Chem Soc Perkin Trans 2:799
Visscher L, Dyall KG (1997) Atom Data Nucl Data Tabl 67:207
Allen MP, Tildeslay DJ (1987) Computer Simulation of Liquids. Clarendon Press, Oxford
Acknowledgments
First of all, we are very grateful for help, valuable discussions and know-how (via providing the ReSpect code) to Vladimír G. Malkin, Oľga L. Malkina (Slovak Academy of Science) and Michal Repiský (University of Tromsø). The financial support was obtained from APVV (contract No. APVV-0202-10) and VEGA (contracts No. 1/0327/12 and 1/0765/14). We are grateful to the HPC center at the Slovak University of Technology in Bratislava, which is a part of the Slovak Infrastructure of High Performance Computing (SIVVP project, ITMS code 26230120002, funded by the European region development funds) for the computational time and resources made available.
Conflict of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary material
Supplementary material contains complete set of geometries from all the 300 K MDSs in the form of xyz files.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malček, M., Bučinský, L., Valko, M. et al. Calculations of hyperfine coupling constant of copper(II) in aqueous environment. Finite temperature molecular dynamics and relativistic effects. J Mol Model 21, 237 (2015). https://doi.org/10.1007/s00894-015-2752-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2752-8