Abstract
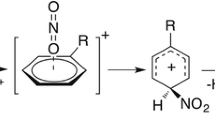
G3(MP2)//B3-CEP theory was applied to study the mechanism of phenol nitration in the gas phase, as promoted by the electrophile NO2 +. The results of studying this mechanism at the G3(MP2)//B3-CEP level pointed to the occurrence of a single-electron transfer (SET) from the aromatic π-system to the nitronium ion prior to σ-complex formation. The formation of an initial π-complex between the nitronium ion and phenol was not observed. Excellent agreement between the activation barriers predicted by G3(MP2)//B3-CEP and those yielded by other, more accurate, versions of the G3 theory showed that the former is a useful tool for studying reaction mechanisms, as G3(MP2)//B3-CEP is much less computationally expensive than other high-level methods.

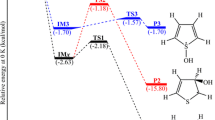
Gibbs free energy diagram for phenol nitration in the gas phase at a temperature of 298.15K calculated at the G3(MP2)//B3-CEP level of theory.










Similar content being viewed by others
References
Taylor R (1990) Electrophilic aromatic substitution, 1st edn. Wiley, Chichester
Cardoso SP, de Carneiro JWM (2001) Nitração aromática: substituição eletrofílica ou reação com transferência de elétrons? Quim Nova 24:381–389. doi:10.1590/S0100-40422001000300015
Olah GA, Malhotra R, Narang SC (1989) Nitration. Methods and mechanisms. VCH, Weinheim
Hughes ED, Ingold CK, Reed RI (1950) Kinetics and mechanism of aromatic nitration. Part II. Nitration by the nitronium ion, NO2 +, derived from nitric acid. J Chem Soc. doi:10.1039/jr9500002400
Wheland GW (1942) A quantum mechanical investigation of the orientation of substituents in aromatic molecules. J Am Chem Soc 64:900–908. doi:10.1021/ja01256a047
Olah GA, Kuhn SJ, Flood SH (1961) Aromatic substitution. VIII. Mechanism of the nitronium tetrafluoroborate nitration of alkylbenzenes in tetramethylene sulfone solution. Remarks on certain aspects of electrophilic aromatic substitution. J Am Chem Soc 83:4571–4580. doi:10.1021/ja01483a017
Dewar MJS (1946) The kinetics of some benzidine rearrangements, and a note on the mechanism of aromatic substitution. J Chem Soc 777–781. doi:10.1039/jr9460000777
Olah GA (1971) Aromatic substitution. XXVIII. Mechanism of electrophilic aromatic substitutions. Acc Chem Res 4:240–248. doi:10.1021/ar50043a002
Kenner J (1946) Oxidation and reduction in chemistry. Nature 157:340–340. doi:10.1038/157340a0
Weiss J (1946) Simple electron transfer processes in systems of conjugated double bonds. Trans Faraday Soc 42:116. doi:10.1039/tf9464200116
Perrin CL (1977) Necessity of electron transfer and a radical pair in the nitration of reactive aromatics. J Am Chem Soc 99:5516–5518. doi:10.1021/ja00458a065
Kochi JK (1992) Inner-sphere electron transfer in organic chemistry. Relevance to electrophilic aromatic nitration. Acc Chem Res 25:39–47. doi:10.1021/ar00013a006
Fukuzumi S, Kochi JK (1981) Electrophilic aromatic substitution. Charge-transfer excited states and the nature of the activated complex. J Am Chem Soc 103:7240–7252. doi:10.1021/ja00414a034
Peluso A, Del Re G (1996) On the occurrence of an electron-transfer step in aromatic nitration. J Phys Chem 100:5303–5309. doi:10.1021/jp9530156
Albunia AR, Borrelli R, Peluso A (2000) The occurrence of electron transfer in aromatic nitration: dynamical aspects. Theor Chem Acc 104:218–222. doi:10.1007/s002140000141
Esteves PM, De M, Carneiro JW, Cardoso SP et al (2003) Unified mechanistic concept of electrophilic aromatic nitration: convergence of computational results and experimental data. J Am Chem Soc 125:4836–4849. doi:10.1021/ja021307w
De Queiroz JF, de Carneiro JWM, Sabino AA et al (2006) Electrophilic aromatic nitration: understanding its mechanism and substituent effects. J Org Chem 71:6192–6203. doi:10.1021/jo0609475
Chen L, Xiao H, Xiao J, Gong X (2003) DFT study on nitration mechanism of benzene with nitronium ion. J Phys Chem A 107:11440–11444. doi:10.1021/jp030167p
Arrieta A, Cossıo FP (2007) Loss of aromaticity and π-electron delocalization in the first step of the electrophilic aromatic nitration of benzene, phenol and benzonitrile. J Mol Struct THEOCHEM 811:19–26. doi:10.1016/j.theochem.2007.03.007
Wheeler SE, Houk KN (2009) Substituent effects in cation/pi interactions and electrostatic potentials above the centers of substituted benzenes are due primarily to through-space effects of the substituents. J Am Chem Soc 131:3126–3127. doi:10.1021/ja809097r
Koleva G, Galabov B, Wu JI et al (2009) Electrophile affinity: a reactivity measure for aromatic substitution. J Am Chem Soc 131:14722–14727. doi:10.1021/ja902194y
Xu XF, Zilberg S, Haas Y (2010) Electrophilic aromatic substitution: the role of electronically excited states. J Phys Chem A 114:4924–4933. doi:10.1021/jp911250g
Hadzic M, Braïda B, Volatron F (2011) Wheland intermediates: an ab initio valence bond study. Org Lett 13:1960–1963. doi:10.1021/ol200327s
Schmitt RJ, Buttrill SE, Ross DS (1984) Gas-phase ion-molecule nitration chemistry: the nitration of aromatic radical cations by nitrogen dioxide. J Am Chem Soc 106:926–930. doi:10.1021/ja00316a017
Aschi M, Attina M, Cacace F, Ricci A (1994) Experimental study on the mechanism of gas-phase aromatic nitration by protonated methyl nitrate. J Am Chem Soc 116:9535–9542. doi:10.1021/ja00100a018
Attinà M, Cacace F, Speranza M (1992) FT-ICR studies of gas-phase ionic nitration of benzene: the role of electron- and proton-transfer processes. Int J Mass Spectrom Ion Proc 117:37–46. doi:10.1016/0168-1176(92)80084-E
Rocha CMR, Pereira DH, Morgon NH, Custodio R (2013) Assessment of G3(MP2)//B3 theory including a pseudopotential for molecules containing first-, second-, and third-row representative elements. J Chem Phys 139:184108. doi:10.1063/1.4826519
Curtiss LA, Redfern PC, Raghavachari K (2005) Assessment of Gaussian-3 and density-functional theories on the G3/05 test set of experimental energies. J Chem Phys 123:124107. doi:10.1063/1.2039080
Curtiss LA, Redfern PC, Raghavachari K et al (1999) Gaussian-3 theory using reduced Møller–Plesset order. J Chem Phys 110:4703. doi:10.1063/1.478385
Baboul AG, Curtiss LA, Redfern PC, Raghavachari K (1999) Gaussian-3 theory using density functional geometries and zero-point energies. J Chem Phys 110:7650. doi:10.1063/1.478676
Wang X, Lau K-C (2012) Theoretical investigations on charge-transfer properties of novel high mobility n-channel organic semiconductors—diazapentacene derivatives. J Phys Chem C 116:22749–22758. doi:10.1021/jp309226z
Silva ALR, Cimas Á, Vale N et al (2013) Experimental and computational study of the energetics of hydantoin and 2-thiohydantoin. J Chem Thermodyn 58:158–165. doi:10.1016/j.jct.2012.10.010
Gao A, Liang X, Li L, Cui J (2013) A Gaussian-3 theoretical study of the alkylthio radicals and their anions: structures, thermochemistry, and electron affinities. J Mol Model 19:3225–3231. doi:10.1007/s00894-013-1855-3
Paine SW, Salam A (2013) Computational study of tautomerism and aromaticity in mono- and dithio-substituted tropolone. Int J Quantum Chem 113:1245–1252. doi:10.1002/qua.24268
Ducati LC, Custodio R, Rittner R (2010) Exploring the G3 method in the study of rotational barrier of some simple molecules. Int J Quantum Chem 110:2006–2014. doi:10.1002/qua.22585
Pereira DH, Ducati LC, Rittner R, Custodio R (2014) A study of the rotational barriers for some organic compounds using the G3 and G3CEP theories. J Mol Model 20:2199. doi:10.1007/s00894-014-2199-3
Kovacevic G, Sabljic A (2013) Theoretical study on the mechanism and kinetics of addition of hydroxyl radicals to fluorobenzene. J Comput Chem 34:646. doi:10.1002/jcc.23175
Mora JR, Lezama J, Berroteran N et al (2012) Density functional theory and ab initio study on the reaction mechanisms of the homogeneous, unimolecular elimination kinetics of selected 1-chloroalkenes in the gas phase. Int J Quantum Chem 112:3729. doi:10.1002/qua.24175
Ali MA, Rajakumar B (2010) Kinetics of OH radical reaction with CF3CHFCH2F (HFC-245eb) between 200 and 400 K: G3MP2, G3B3 and transition state theory calculations. J Mol Struct Theochem 949:73. doi:10.1016/j.theochem.2010.03.006
Stevens WJ, Basch H, Krauss M (1984) Compact effective potentials and efficient shared-exponent basis sets for the first- and second-row atoms. J Chem Phys 81:6026. doi:10.1063/1.447604
Stevens WJ, Krauss M, Basch H, Jasien PG (1992) Relativistic compact effective potentials and efficient, shared-exponent basis sets for the third-, fourth-, and fifth-row atoms. Can J Chem 70:612–630. doi:10.1139/v92-085
Pereira DH, Ramos AF, Morgon NH, Custodio RR (2011) Implementation of pseudopotential in the G3 theory for molecules containing first-, second-, and non-transition third-row atoms. J Chem Phys 135:034106. doi:10.1063/1.3609241
Pereira DH, Ramos AF, Morgon NH, Custodio RR (2011) Erratum: “Implementation of pseudopotential in the G3 theory for molecules containing first-, second-, and non-transition third-row atoms” [J. Chem. Phys. 135, 034106 (2011)]. J Chem Phys 135:219901. doi:10.1063/1.3666235
Curtiss LA, Redfern PC, Raghavachari K (2011) G n theory. Wiley Interdiscip Rev Comput Mol Sci 1:810. doi:10.1002/wcms.59
Fukui K (1981) The path of chemical reactions—the IRC approach. Acc Chem Res 14:363. doi:10.1021/ar00072a001
Šakić D, Vrček V (2012) Prereactive complexes in chlorination of benzene, triazine, and tetrazine: a quantum chemical study. J Phys Chem A 116:1298. doi:10.1021/jp210993k
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735. doi:10.1063/1.449486
Glendening ED, Reed AE, Carpenter JE, Weinhold F (2009) program: NBO analysis, version 5.1
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09. Gaussian, Inc., Wallingford
Herzberg G (1966) Molecular spectra and molecular structure: electronic spectra and electronic structure of polyatomic molecules. Van Nostrand, New York
Chen Z, Mo Y (2013) Electron transfer in electrophilic aromatic nitration and nitrosation: computational evidence for the Marcus inverted region. J Chem Theory Comput 9:4428. doi:10.1021/ct400618k
Vogel AI, Tatchell AR, Furnis BS et al (1989) Vogel’s textbook of practical organic chemistry, 5th edn. Longman, London
Acknowledgments
We acknowledge financial support from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo—Center for Computational Engineering and Sciences, grant 2013/08293-7), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), and FAEPEX-UNICAMP (Fundo de Apoio ao Ensino, à Pesquisa e à Extensão da UNICAMP). The National Center of High Performance Computing in São Paulo (CENAPAD–SP) is acknowledged for making its computational facilities available to us.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
G3(MP2)//B3-CEP molecular energies, thermal corrections to 298.15 K, and geometric parameters for the optimized structures calculated at B3LYP/CEP-P31G(d) level. For transition structures in which imaginary frequencies were observed, displacement vectors of the imaginary normal modes are shown. (DOCX 322 kb)
Rights and permissions
About this article
Cite this article
Rocha, C.M.R., Rodrigues, J.A.R., Moran, P.J.S. et al. An interpretation of the phenol nitration mechanism in the gas phase using G3(MP2)//B3-CEP theory. J Mol Model 20, 2524 (2014). https://doi.org/10.1007/s00894-014-2524-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2524-x