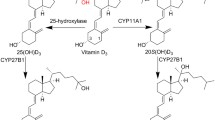
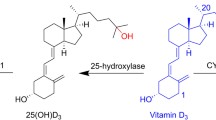
Abstract
1α,25(OH)2D3, which is directly mediated by the vitamin D receptor (VDR), exerts a wide variety of biological actions. However, the treatment with 1α,25(OH)2D3 is limited because of its side effects. Many analogs and several nonsteroidal mimics with potent biological activity have been reported so far, and our rationale for designing the VDR agonists was on the basis of computer-aided drug design method by de novo design of A-ring and C/D-ring position of 1α,25(OH)2D3. Pyrimidine-2,4-diamine was selected as A-ring, and naphthalene and benzene were chosen as C/D-ring. By linking different components, a virtue compound library was obtained. To evaluate the contribution to activity of each component, we performed a series of automated molecular docking operations. Results revealed that the 19-dimethyl derivatives (the C-19 position correspond to C-20 in 1α,25(OH)2D3) show the favorable docking affinity to VDR. Moreover, the docking results are quite robust when further validated by molecular dynamics simulations. In addition, by free energy analysis using molecular mechanics-Poisson-Boltzmann surface area (MM-PBSA) method, the driving force of the binding between VDR and the ligands is proved to be hydrophobic interactions. Thus, a possible strategy to design new series of VDR agonists is proposed. The strategy can be successfully applied to explain the high potential activities of the 19-dimethyl derivatives. It is anticipated that the findings reported here may provide useful information for designing effective VDR agonists as well as the therapeutic treatment of VDR-related diseases.






Similar content being viewed by others
References
Bouillon R, Okamura WH, Norman AW (1995) Structure-function relationships in the vitamin D endocrine system. Endocr Rev 16:200–257
Norman AW, Frankel JB, Heldt AM, Grodsky GM (1980) Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 209:823–825
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumgerg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839
Nakano Y, Kato Y, Imai K, Ochiai E, Namekawa J, Ishizuka S, Takenouchi K, Tanatani A, Hashimoto Y, Nagasawa K (2006) Practical synthesis and evaluation of the biological activities of 1alpha,25-dihydroxyvitamin D3 antagonists, 1alpha,25-dihydroxyvitamin D3-26,23-lactams. Designed on the basis of the helix 12-folding inhibition hypothesis. J Med Chem 49:2398–2406
Evans RM (1988) The steroid and thyroid hormone receptor superfamily. Science 240:889–895
Peleg S, Posner GH (2003) Vitamin D analogs as modulators of vitamin D receptor action. Curr Top Med Chem 3:1555–1572
Aranda A, Pascual A (2001) Nuclear hormone receptors and gene expression. Physiol Rev 81:1269–1304
Brown AJ (2000) Mechanisms for the selective actions of vitamin D analogues. Curr Pharm Des 6:701–706
Masuda S, Jones G (2003) Vitamin D analogs-drug design based on proteins involved in vitamin D signal transduction. Curr Drug Targets Immune Endocr Metab Disord 3:43–66
Sicinski RR, Glebocka A, Plum LA, DeLuca HF (2007) Design, synthesis, and biological evaluation of a 1α,25-Dihydroxy-19-norvitamin D3 analogue with a frozen a-ring conformation. J Med Chem 50:6154–6164
Bouillon R, Sarandeses LA, Allewaert K, Zhao J, Mascarenas JL, Mourino A, Vrielynck S, de Clercq P, Vandewalle M (1993) Biologic activity of dihydroxylated 19-nor-(pre)vitamin D3. J Bone Miner Res 8:1009–1015
Okamura WH, Aurrecoechea JM, Gibbs RA, Norman AW (1989) Synthesis and biological activity of 9,11-Dehydrovitamin D3 analogues: stereoselective preparation of 6β-Vitamin D vinylallenes and a concise enynol synthesis for preparing the A-ring. J Org Chem 54:4072–4083
Wang YZ, Li H, Bruns ME, Uskokovic M, Truitt GA, Horst R, Reinhardt T, Christakos S (1993) Effect of 1,25,28-trihydroxyvitamin D2 and 1,24,25-trihydroxyvitamin D3 on intestinal calbindin-D9K mRNA andprotein: Is there a correlation with intestinal calciumtransport? J Bone Miner Res 8:1483–1490
Riveiros R, Rumbo A, Sarandeses LA, Mouriño A (2007) Synthesis and Conformational Analysis of 17α,21-Cyclo-22-Unsaturated Analogues of Calcitriol. J Org Chem 72:5477–5485
Yamada S, Yamamoto K, Masuno H, Ohta M (1998) Conformation-function relationship of vitamin D: conformational analysis predicts potential side-chain structure. J Med Chem 41:1467–1475
Westermann J, Schneider M, Platzek J, Petrov O (2007) Practical synthesis of a heterocyclic immunosuppressive vitamin D analogue. Org Process Res Dev 11:200–205
Bury Y, Ruf D, Hansen CM, Kissmeyer AM, Binderup L, Carlberg C (2001) Molecular evaluation of vitamin D3 receptor agonists designed for topical treatment of skin diseases. J Invest Dermatol 116:785–792
Inaba Y, Yoshimoto N, Sakamaki Y, Nakabayashi M, Ikura T, Tamamura H, Ito N, Shimizu M, Yamamoto K (2009) A new class of vitamin D analogues that induce structural rearrangement of the ligand-binding pocket of the receptor. J Med Chem 52:1438–1449
Verstuyf A, Verlinden L, van Etten E, Shi L, Wu Y, D'Halleweyn C, van Haver D, Zhu GD, Chen YJ, Zhou X, Haussler MR, De Clercq P, Vandewalle M, van Baelen H, Mathieu C, Bouillon R (2000) Biological activity of CD-ring modified 1α,25-dihydroxyvitamin D analogues: C-ring and five-membered D-ring analogues. J Bone Miner Res 15:237–252
Hosoda S, Tanatani A, Wakabayashi K, Makishima M, Imai K, Miyachi H, Nagasawad K, Hashimoto Y (2006) Ligands with a 3,3-diphenylpentane skeleton for nuclear vitamin D and androgen receptors: dual activities and metabolic activation. Bioorg Med Chem 14:5489–5502
Miuraa D, Manabeb K, Gao QZ, Normanc AW, Ishizukab S (1999) 1α,25-Dihydroxyvitamin D3-26,23-lactone analogs antagonize differentiation of human leukemia cells (HL-60 cells) but not of human acute promyelocytic leukemia cells (NB4 cells). FEBS Lett 460:297–302
Bury Y, Steinmeyer A, Carlberg C (2000) Structure activity relationship of carboxylic ester antagonists of the vitamin D(3) receptor. Mol Pharmacol 58:1067–1074
García-Sosa AT, Mancera RL (2006) The effect of a tightly bound water molecule on scaffold diversity in the computer-aided de novo ligand design of CDK2 inhibitors. J Mol Model 12:422–431
Francis SM, Mittal A, Sharma M, Bharatam PV (2008) Design of Benzene-1,2-diamines as selective inducible nitric oxide synthase inhibitors: a combined de novo design and docking analysis. J Mol Model 14:215–224
Böhm HJ (1994) On the use of LUDI to search the fine chemicals directory for ligands of proteins of known three-dimensional structure. J Comput Aid Mol Des 8:623–632
Huang RB, Du QS, Wang CH, Chou KC (2008) An in-depth analysis of the biological functional studies based on the NMR M2 channel structure of influenza A virus. Biochem Biophys Res Commun 377:1243–1247
Du QS, Huang RB, Wang CH, Li XM, Chou KC (2009) Energetic analysis of the two controversial drug binding sites of the M2 proton channel in influenza A virus. J Theor Biol 259:159–164
Wang SQ, Du QS, Chou KC (2007) Study of drug resistance of chicken influenza a virus (H5N1) from homology-modeled 3D structures of neuraminidases. Biochem Biophys Res Commun 354:634–640
Wang SQ, Du QS, Huang RB, Zhang DW, Chou KC (2009) Insights from investigating the interaction of oseltamivir (Tamiflu) with neuraminidase of the 2009 H1N1 swine flu virus. Biochem Biophys Res Commun 386:432–436
Norman AW, Myrtle JF, Miogett RJ, Nowicki HG, Williams V, Popjaák G (1971) 1,25-Dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine. Science 173:51–54
Verlinden L, Verstuyf A, Van Camp M, Marcelis S, Sabbe K, Zhao XY, De Clercq P, Vandewalle M, Bouillon R (2000) Two novel 14-epi-analogues of 1,25-dihydroxyvitamin D3 inhibit the growth of human breast cancer cells in vitro and in vivo. Cancer Res 60:2673–2679
Hansen CM, Hamberg KJ, Binderup E, Binderup L (2000) Seocalcitol (EB 1089) A vitamin D analogue of anti-cancer potential. Background, design, synthesis, pre-clinical and clinical evaluation. Curr Pharm Des 26:803–828
Hisatake J, O'Kelly J, Uskokovic MR, Tomoyasu S, Koeffler HP (2001) Novel vitamin D3 analog, 21-(3-methyl-3-hydroxy-butyl)-19-nor D3, that modulates cell growth, differentiation, apoptosis, cell cycle, and induction of PTEN in leukemic cells. Blood 97:2427–2433
Kissmeyer AM, Binderup L (1991) Calcipotriol (MC 903): pharmacokinetics in rats and biological activities of metabolites. A comparative study with 1,25(OH)2D3. Biochem Pharmacol 41:1601–1606
Huey R, Morris GM, Olson AJ, Goodsell DS (2007) A semiempirical free energy force field with charge-based desolvation. J Comput Chem 28:1145–1152
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM Jr, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc 117:5179–5197
Wang JM, Wolf RM, Caldwell JW, Kollamn PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25:1157–1174
Ryckaert JP, Ciccotti G, Berendsen HJC (1977) Numerical integration of the Cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23:327–341
Darden T, York D, Pedersen L (1993) Particle mesh ewald: an N-log(N) method for ewald sums in large systems. J Chem Phys 98:10089–10092
Honig B, Nicholls A (1995) Classical electrostatics in biology and chemistry. Science 268:1144–1149
Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L, Lee M, Lee T, Duan Y, Wang W, Donini O, Cieplak P, Srinivasan J, Case DA, Cheatham TE (2000) Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res 33:889–897
Massova I, Kollman PA (2000) Combined molecular mechanical and continuum solvent approach (MM PBSA/GBSA) to predict ligand binding. Perspect Drug Discov Des 18:113–135
Onufriev A, Bashford D, Case DA (2004) Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins 55:383–394
Zhou ZG, Madrid M, Evanseck JD, Madura JD (2005) Effect of a bound non-nucleoside RT inhibitor on the dynamics of wild-type and mutant HIV-1 reverse transcriptase. J Am Chem Soc 127:17253–17260
Bissantz C, Folkers G, Rognan D (2000) Protein-based virtual screening of chemical databases. 1. Evaluation of different docking/scoring combinations. J Med Chem 43:4759–4767
Wang R, Lu Y, Wang S (2003) Comparative evaluation of 11 scoring functions for molecular docking. J Med Chem 46:2287–2303
Plummer MS, Holland DR, Shahripour A, Lunney EA, Fergus JH, Marks JS, McConnell P, Mueller WT, Sawyer TK (1997) Design, synthesis, and cocrystal structure of a nonpeptide Src SH2 domain ligand. J Med Chem 40:3719–3725
Yamamoto K, Abe D, Yoshimoto N, Choi M, Yamagishi K, Tokiwa H, Shimizu M, Makishima M, Yamada S (2006) Vitamin D receptor: ligand recognition and allosteric network. J Med Chem 49:1313–1324
Yamagishia K, Tokiwaa H, Makishimac M, Yamada S (2010) Interactions between 1α,25(OH)2D3 and residues in the ligand-binding pocket of the vitamin D receptor: a correlated fragment molecular orbital study. J Steroid Biochem 121:63–67
Acknowledgments
We gratefully acknowledge the support from Project of Undergraduate Educational Reform & Capacities in Tianjin University (No. 200904050).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(PDF 191 KB)
Rights and permissions
About this article
Cite this article
Shen, XL., Takimoto-Kamimura, M., Wei, J. et al. Computer-aided de novo ligand design and docking/molecular dynamics study of Vitamin D receptor agonists. J Mol Model 18, 203–212 (2012). https://doi.org/10.1007/s00894-011-1066-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-1066-8