Abstract
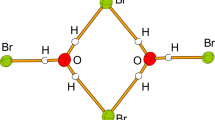
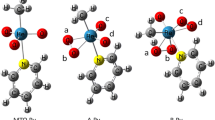
Reactions of lithium halide (LiX, X = F, Cl, Br and I) and methyl halide (CH3X, X = F, Cl, Br and I) have been investigated at the B3LYP/6-31G(d) level of theory using the microhydration model. Beginning with hydrated lithium ion, four or two water molecules have been conveniently introduced to these aqueous-phase halogen-exchange SN2 reactions. These water molecules coordinated with the center metal lithium ion, and also interacted with entering and leaving halogen anion via hydrogen bond in complexes and transition state, which to some extent compensated hydration of halogen anion. At 298 K the reaction profiles all involve central barriers ΔE cent which are found to decrease in the order F > Cl > Br > I. The same trend is also found for the overall barriers (ΔE ovr ) of the title reaction. In the SN2 reaction of sodium iodide and methyl iodide, the activation energy agrees well with the aqueous conductometric investigation.








Similar content being viewed by others
References
For experimental investigation on aqueous-phase SN2 reactions, see a review: Parker AJ (1969) Chem Rev 69:1–32 and its cited references
Dewar MJS, Dougherty RC (1975) The PMO theory of organic chemistry. Plenum, New York, p 234
Arnett EM, Johnston DE, Small LE (1975) J Am Chem Soc 97:5598–5600
Olmstead WN, Brauman JI (1977) J Am Chem Soc 99:4219–4228
DePuy CH, Gronert S, Mullin A, Bierbaum VM (1990) J Am Chem Soc 112:8650–8655
Pellerite MJ, Brauman JI (1983) J Am Chem Soc 105:2672–2680
Caldwell G, Magnera TF, Kebarle P (1984) J Am Chem Soc 106:959–966
Shi Z, Boyd RJ (1990) J Am Chem Soc 112:6789–6796
Glukhovtsev MN, Pross A, Radom L (1995) J Am Chem Soc 117:2024–2032
Glukhovtsev MN, Bach RD, Pross A, Radom L (1996) Chem Phys Lett 260:558–564
Glukhovtsev MN, Pross A, Schlegel HB, Bach RD, Radom L (1996) J Am Chem Soc 118:11258–11264
Glad SS, Jensen F (1997) J Am Chem Soc 199:227–232
Cossi M, Adamo C, Barone V (1998) Chem Phys Lett 297:1–7
Safi B, Choho K, Geerlings P (2001) J Phys Chem A 105:591–601
Kato S, Davico GE, Lee HS, DePuy CH (2001) Int J Mass Spectrom 210–211:223–229
Harder S, Streitwieser A, Petty JT, Schleyer PvR (1995) J Am Chem Soc 117:3253–3259
Streitwieser A, Choy GSC, Abu-Hasanayn F (1997) J Am Chem Soc 119:5013–5019
Xiong Y, Zhu HJ, Ren Y (2003) J Mol Struct THEOCHEM 664–665:279–289
Ren Y, Chu SY (2004) J Comput Chem 25:461–467
Hasanayn F, Streitwieser A, Al-Rifai R (2005) J Am Chem Soc 127:2249–2255
Ingold CK (1969) Structure and mechanism in organic chemistry. Cornell University Press, Ithaca, p 457
Westaway KC (1978) Can J Chem 56:2691–2699
Westaway KC, Lai ZG (1989) Can J Chem 67:345–349
Streitwieser A, Jayasree EG (2007) J Org Chem 72:1785–1798
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1988) Phys Rev A 38:3098–3100
Miehlich B, Savin A, Stoll H, Preuss H (1989) Chem Phys Lett 157:200–206
Becke AD (1993) J Chem Phys 98:5648–5652
Wadt WR, Hay PJ (1985) J Chem Phys 82:284–298
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735–746
Foster JP, Weinhold F (1980) J Am Chem Soc 102:7211–7218
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527
Frisch MJ, Trucks GW, Schlegel HB et al (2004) Gaussian 03, Revision E.01. Gaussian, Wallingford
Michaellian KH, Moskovits M (1978) Nature 273:135–136
Egawa T, Yamamoto S, Nakata M, Kuchitsu K (1987) J Mol Struct 156:213–228
Jensen T, Brodersen S, Guelachvili G (1981) J Mol Spectrosc 88:378–393
Graner G (1981) J Mol Spectrosc 90:394–438
Harmony MD, Laurie VW, Kuczkowski RL, Ramsay DA, Lovas FJ, Lafferty WJ, Maki AG (1979) J Phys Chem Ref Data 8:619–711
For the data, see: Allen LC (1989) J Am Chem Soc 111:9003–9014
Lias SG, Bartmess JE, Liebman JF, Holmes JL, Levin RD, Mallard WG (1988) J Phys Chem Ref Data 17 Suppl. 1
Han CC, Dodd JA, Brauman JI (1986) J Phys Chem 90:471–477
Acknowledgments
We thank Scientific Research Funding of Chongqing University, Innovative Talent Training Project of Chongqing University, the Third Stage of “211 project” (No. S-09103), Chongqing Municipal Education Commission (No. KJ-091201) and Bureau of Education of Sichuan Province (No. 2006ZD051) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, S., Xiong, Y. & Wang, J. Theoretical studies on identity SN2 reactions of lithium halide and methyl halide: A microhydration model. J Mol Model 16, 1931–1937 (2010). https://doi.org/10.1007/s00894-010-0688-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0688-6