Abstract
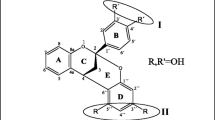
The stereochemistry of A-type dimeric proanthocyanidins was studied, focusing on the factors that determine it, and the changes that occur with R = OCH3, R′ = H, and R = OH, R′ = H as substituents, starting with the study of the conformational space of each species. Using molecular dynamics at a semiempirical level, and complementing with functional density calculations, two conformers of lowest energy were characterized for R = H, eight conformers for R = OH, and three conformers for R = OCH3. Electronic distributions were analyzed at a higher calculation level, thus improving the basis set. Intramolecular interactions were examined and characterized by the theory of atoms in molecules (AIM). Detailed natural bond orbitals (NBO) analysis allowed the description of subtle stereoelectronic aspects of fundamental importance for understanding the stabilization and antioxidant function of these structures. The study was enriched by a deep analysis of maps of molecular electrostatic potential (MEP). The coordinated analysis of MEP, together with the NBO and AIM results, allowed us to rationalize novel distribution aspects of the potential created in the space around a molecule.







Similar content being viewed by others
References
Pomilio A, Müller O, Schilling G, Weinges K (1977) Zur Kenntnis der Proanthocyanidine, XXII. Über die Konstitution der Kondensationsprodukte von Phenolen mit Flavyliumsalzen. Justus Liebigs Ann Chem :597-601
Ricardo da Silva JM, Darmon N, Fernandez Y, Mitjavilla S (1991) Oxygen free radical scavenger capacity in aqueous models of different procyanidins from grape seeds. J Agric Food Chem 39:1549–1552
Liu ZQ, Ma LP, Zhou B, Yang L, Liu ZL (2000) Antioxidative effects of green tea polyphenols on free radical initiated and photosensitized peroxidation of human low density lipoprotein. Chem Phys Lipids 106:53–63
Liu L, Xie B, Cao S, Yang E, Xu X, Guo S (2007) A-type procyanidins from Litchi chinensis pericarp with antioxidant activity. Food Chem 105:1446–1451
Kedage VV, Tilak JC, Dixit GB, Devasagayam TPA, Mhatre M (2007) A study of antioxidantproperties of some varieties of grapes (Vitis vinifera L). Crit Rev Food Sci Nutr 47:175–185
Pinent M, Bladé C, Salvadó MJ, Blay M, Pujadas G, Fernandez-Larrea J, Arola L, Ardevol A (2006) Procyanidin effects on adipocyte-related pathologies. Crit Rev Food Sci Nutr 46:543–550
Pomilio A, Ellmann B, Künstler K, Schilling G, Weinges K (1977) Naturstoffe aus Arzneipflanzen, XXI. 13C-NMR-spektroskopische Untersuchungen an Flavanoiden. Justus Liebigs Ann Chem :588–596
Lobayan RM, Jubert AH, Pomilio AB (2009) Conformational and electronic (AIM/NBO) study of unsubstituted A-type dimeric Proanthocyanidin. J Mol Model 15:537–550
HyperChem Release 7.5, Hypercube Inc, Gainsville, FL
Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Revision A.7. Gaussian Inc, Pittsburgh
Flúkiger P, Lúthi HP, Portmann S, Weber J (2000) MOLEKEL 4.0. Swiss Center for Scientific Computing, Manno, Switzerland
Biegler-Koning FW, Bader RFW, Tang TH (1982) Calculation of the average properties of atoms in molecules.II. J Comput Chem 3:317–328
Glendening ED, Reed AE, Carpenter JE, Weinhold F NBO 3.1. Program as implemented in the Gaussian 98 package
Bader RFW (1995) Atoms in molecules—a quantum theory. Oxford University Press, Oxford
Bader RFW (1990) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Bader RFW (1998) A Bond Path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323
Popelier PLA (1998) Characterization of a dihydrogen bond on the basis of the electron density. J Phys Chem A 102:1873–1878
Carroll MT, Bader RFW (1988) An analysis of the hydrogen bond in BASE-HF complexes using the theory of atoms in molecules. Mol Phys 65:695–722
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, New York
Koch U, Popelier PLA (1995) Characterization of C–H–O hydrogen bonds on the basis of the charge density. J Phys Chem 99:9747–9754
Lee JC, Peris E, Rheingod AL, Crabtree CH (1994) An unusual type of H⋯H interaction: Ir-H⋯H-O and Ir-H⋯H–N hydrogen bonding and its involvement in sigma-bond metathesis. J Am Chem Soc 116:11014–11019
Pakiari AH, Mohajeri A (2003) Multi dihydrogen bonds. J Mol Struct Theochem 620:31–36
Grabowski SJ, Sokalski WA, Leszczynski J (2007) Wide spectrum of H⋯H interactions; van der Waals contacts, dihydrogen bonds and covalency. Chem Phys 337:68–76
Matta CF (2006) The non-electrostatic limit of closed-shell interaction between two hydrogen atoms. A critical review. In: Grabowski SJ (ed) Hydrogen bonding–new insights. Springer, Netherlands, pp 337–375
Pacios LF (2006) Changes of electron properties in the formation of hydrogen bonds. In: Grabowski SJ (ed) Hydrogen bonding–new insights. Springer, Netherlands, pp 109–148
Politzer P, Truhlar DG (eds) (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, NY
Politzer P, Landry SJ, Warnheim T (1982) Proposed procedure for using electrostatic potentials to predict and interpret nucleophilic processes. J Phys Chem 86:4767–4771
Politzer P, Abrahmsen L, Sjoberg P (1984) Effects of amino and nitro substituents upon the electrostatic potential of an aromatic ring. J Am Chem Soc 106:855–860
Politzer P, Laurence PR, Jayasuriya K (1985) Molecular electrostatic potentials: an effective tool for the elucidation of biochemical phenomena. Environ Health Perspect 61:191–202
Politzer P, Murray JS (1991) Theoretical biochemistry and molecular biophysics: a comprehensive survey, vol 2. In: Beveridge DL, Lavery R (eds) Protein. Adenine , Schenectady, pp 165–191
Murray JS, Lane P, Brinck T, Politzer P, Sjoberg P (1991) Electrostatic potentials on the molecular surfaces of cyclic ureides. J Phys Chem 95:844–848
Muñoz-Caro C, Niño A, Sement ML, Leal JM, Ibeas S (2000) Modeling of protonation processes in acetohydroxamic acid. J Org Chem 65:405–410
Politzer P, Murray J, Concha MC (2002) The complementary roles of molecular surface electrostatic potentials and average local ionization energies with respect to electrophilic processes. Int J Quantum Chem 88:19–27
Acknowledgments
Thanks are due to Agencia de Promoción Científica y Tecnológica Argentina (MINCYT), CONICET, Universidad Nacional de La Plata and Universidad de Buenos Aires (Argentina) for financial support. A.B.P. is a Senior Research Member of the National Research Council of Argentina (CONICET). A.H.J. is Member of the Scientific Research Career (CIC, Provincia de Buenos Aires). E.N.B. acknowledges a fellowship (IP-PRH No 54) from Agencia de Promoción Científica y Tecnológica Argentina and Universidad de la Cuenca del Plata (Corrientes, Argentina). R.M.L. acknowledges Universidad de la Cuenca del Plata for facilities provided during the course of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 511 kb)
ESM Fig. S1a–d
Maps of molecular electrostatic potential (MEP) for Z1 rotamers, substituted with R = OCH3 (a), R = H (b) and R = OH (c) and for Z2 rotamers substituted with R = OH (d) of A-type dimeric proanthocyanidins, in a.u. Oblique lines indicate the decreasing reactivity towards electrophilic attack OCH3 (a) >H (b) >OH (c). Horizontal lines show higher reactivity of Z1 (c) rotamers than that of Z2 rotamers (d). (PDF 797 kb)
Rights and permissions
About this article
Cite this article
Bentz, E.N., Jubert, A.H., Pomilio, A.B. et al. Theoretical study of Z isomers of A-type dimeric proanthocyanidins substituted with R=H, OH and OCH3: stability and reactivity properties. J Mol Model 16, 1895–1909 (2010). https://doi.org/10.1007/s00894-010-0682-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0682-z