Abstract
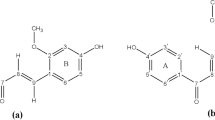
The conformational space of the unsubstituted A-type dimeric proanthocyanidin was scanned using molecular dynamics at a semiempirical level, and complemented with functional density calculations. The lowest energy conformers were obtained. Electronic distributions were analysed at a higher calculation level, thus improving the basis set. A topological study based on Bader’s theory (AIM: atoms in molecules) and natural bond orbital (NBO) framework was performed. Furthermore, molecular electrostatic potential maps (MEPs) were obtained and analysed. NMR chemical shifts were calculated at ab initio level and further compared with previous experimental values; coupling constants were also calculated. The stereochemistry of the molecule is thoroughly discussed, revealing the key role that hyperconjugative interactions play in defining experimental trends. These results show the versatility of geminal spin–spin coupling 2J(C-1′,O) as a probe for stereochemical studies of proanthocyanidins.






Similar content being viewed by others
References
Pomilio A, Müller O, Schilling G, Weinges K (1977) Zur Kenntnis der Proanthocyanidine, XXII. Über die Konstitution der Kondensationsprodukte von Phenolen mit Flavyliumsalzen. Justus Liebigs Ann Chem, 597–601. doi:10.1002/jlac.197719770409
Ricardo da Silva JM, Darmon N, Fernandez Y, Mitjavilla S (1991) Oxygen free radical scavenger capacity in aqueous models of different procyanidins from grape seeds. J Agric Food Chem 39:1549–1552. doi:10.1021/jf00009a002
Liu ZQ, Ma LP, Zhou B, Yang L, Liu ZL (2000) Antioxidative effects of green tea polyphenols on free radical initiated and photosensitized peroxidation of human low density lipoprotein. Chem Phys Lipids 106:53–63. doi:10.1016/S0009-3084(00)00133-X
Liu L, Xie B, Cao S, Yang E, Xu X, Guo S (2007) A-type procyanidins from Litchi chinensis pericarp with antioxidant activity. Food Chem 105:1446–1451. doi:10.1016/j.foodchem.2007.05.022
Kedage VV, Tilak JC, Dixit GB, Devasagayam TPA, Mhatre M (2007) A study of antioxidant properties of some varieties of grapes (Vitis vinifera L.). Crit Rev Food Sci Nutr 47:175–185. doi:10.1080/10408390600634598
Pinent M, Bladé C, Salvadó MJ, Blay M, Pujadas G, Fernandez-Larrea J, Arola L, Ardevol A (2006) Procyanidin effects on adipocyte-related pathologies. Crit Rev Food Sci Nutr 46:543–550. doi:10.1080/10408390500354537
Pomilio A, Ellmann B, Künstler K, Schilling G, Weinges K (1977) Naturstoffe aus Arzneipflanzen, XXI. 13C-NMR-spektroskopische Untersuchungen an Flavanoiden. Justus Liebigs Ann Chem, 588–596. doi:10.1002/jlac.197719770408
Ditchfield R, Miller DP, Pople JA (1970) Molecular orbital theory of carbon NMR chemical shifts. Chem Phys Lett 6:573–575. doi:10.1016/0009-2614(70)85229-0
Gelius U, Roos B, Siegbahn P (1970) Ab initio MO SCF calculations of ESCA shifts in sulphur-containing molecules. Chem Phys Lett 4:471–475. doi:10.1016/0009-2614(70)85018-7
Ditchfield R (1972) On molecular orbital theories of NMR chemical shifts. Chem Phys Lett 15:203–206. doi:10.1016/0009-2614(72)80149-0
Kaupp M, Malkin VG, Malkina OL, Salahub DR (1996) Ab initio ECP/DFT calculation and interpretation of carbon and oxygen nmr chemical shift tensors in transition-metal carbonyl complexes. Chem Eur J 2:24–30. doi:10.1002/chem.19960020108
Schindler M, Kutzelnigg W (1983) Theory of magnetic susceptibilities and NMR chemical shifts in terms of localized quantities. 3. Application to hydrocarbons and other organic molecules. J Am Chem Soc 105:1360–1370. doi:10.1021/ja00343a049
Gauss J (1993) Effects of electron correlation in the calculation of nuclear magnetic resonance chemical shifts. J Chem Phys 99:3629–3643. doi:10.1063/1.466161
van Wüllen CJ (1995) Chem Phys 102:2806–2811. doi:10.1063/1.468657
Bader RFW (1990) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928. doi:10.1021/cr00005a013
Bader RFW (1995) Atoms in molecules. A quantum theory. Oxford University Press, Oxford
Popelier P (2000) Atoms in molecules. An introduction. Prentice Hall
HyperChem Release 7.5, Hypercube Inc., USA
Gaussian 98, Revision A.7, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, RobbMA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian, Inc., Pittsburgh PA.
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Flúkiger P, Lúthi HP, Portmann S, Weber J (2000) MOLEKEL 4.0. Swiss Center for Scientific Computing, Manno, Switzerland
Biegler-König FW, Bader RFW, Tang TH (1982) Calculation of the average properties of atoms in molecules. II. J Comput Chem 3:317–328. doi:10.1002/jcc.540030306
Glendening ED, Reed AE, Carpenter JE, Weinhold F NBO 3.1. Program as implemented in the Gaussian 98 package
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397–5403. doi:10.1063/1.458517
Bader RFW (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323. doi:10.1021/jp981794v
Politzer P, Laurence PR, Jayasuriya K (1985) Molecular electrostatic potentials: an effective tool for the elucidation of biochemical phenomena. Environ Health Perspect 61:191–202. doi:10.2307/3430072
Politzer P, Murray JS (1991) In: Beveridge DL, Lavery R (eds) Theoretical biochemistry and molecular biophysics: a comprehensive survey, vol 2, protein. Adenine, Schenectady, NY, Chapter 13
Politzer P, Truhlar DG (eds) (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York
Politzer P, Landry SJ, Waernheim T (1982) Proposed procedure for using electrostatic potentials to predict and interpret nucleophilic processes. J Phys Chem 86:4767–4771
Politzer P, Abrahmsen L, Sjoberg P (1984) Effects of amino and nitro substituents upon the electrostatic potential of an aromatic ring. J Am Chem Soc 106:855–860
Politzer P, Laurence PR, Abrahmsen L, Zilles BA, Sjoberg P (1984) Chem Phys Lett 111:75–78
Murray JS, Lane P, Brinck T, Politzer P, Sjoberg P (1991) Electrostatic potentials on the molecular surfaces of cyclic ureides. J Phys Chem 95:844–848
Bader RFW (1990) Chem Rev 91:893–928. doi:10.1021/cr00005a013
Muñoz-Caro C, Niño A, Sement ML, Leal JM, Ibeas S (2000) Modeling of protonation processes in acetohydroxamic acid. J Org Chem 65:405–410
Alabugin IV, Manoharan M, Peabody S, Weinhold F (2003) Electronic basis of improper hydrogen bonding: a subtle balance of hyperconjugation and rehybridization. J Am Chem Soc 125:5973–5987
Tormena CF, Rittner R, Contreras RH, Peralta JE (2003) Theoretical calculation of proto-proton NMR indirect spin-spin coupling constants in heterocyclic three-membered rings. Ann Magn Reson 2:70–72
Contreras RH, Peralta JE (2000) Angular dependence of spin-spin coupling constants. Prog Nucl Magn Reson Spectrosc 37:321–425. doi:10.1016/S0079-6565(00)00027-3
Acknowledgements
Thanks are due to CONICET, Universidad Nacional de La Plata and Universidad de Buenos Aires (Argentina) for financial support. One of us (A.B.P.) is a Senior Research Member of the National Research Council of Argentina (CONICET). A.H.J. is a Member of the Scientific Research Career (CIC, Provincia de Buenos Aires). R.M.L. acknowledges Universidad de la Cuenca del Plata (Corrientes, Argentina) for facilities provided during the course of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lobayan, R.M., Jubert, A.H., Vitale, M.G. et al. Conformational and electronic (AIM/NBO) study of unsubstituted A-type dimeric proanthocyanidin. J Mol Model 15, 537–550 (2009). https://doi.org/10.1007/s00894-008-0389-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-008-0389-6