Abstract
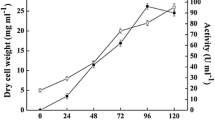
The genome of the hyperthermophilic archaeon Pyrobaculum calidifontis contains an open reading frame, Pcal_1032, annotated as glucokinase. Amino acid sequence analysis showed that Pcal_1032 belonged to ROK (repressor, open reading frame, and kinase) family of sugar kinases. To examine the properties of Pcal_1032, the coding gene was cloned and expressed in Escherichia coli. However, expression of the gene was low resulting in a poor yield of the recombinant protein. A single site directed mutation in Pcal_1032 gene, without altering the amino acid sequence, resulted in approximately tenfold higher expression. Purified recombinant Pcal_1032 efficiently phosphorylated various hexoses with a marked preference for glucose. ATP was the most preferred phosphoryl group donor. Optimum temperature and pH for the glucokinase activity of Pcal_1032 were 95 °C and 8.5, respectively. Catalytic efficiency (k cat/K m) towards glucose was 437 mM−1 s−1. The recombinant enzyme was highly stable against temperature with a half-life of 25 min at 100 °C. In addition, Pcal_1032 was highly stable in the presence of denaturants. There was no significant change in the CD spectra and enzyme activity of Pcal_1032 even after overnight incubation in the presence of 8 M urea. To the best of our knowledge, Pcal_1032 is the most active and highly stable glucokinase characterized to date from archaea, and this is the first description of the characterization of a glucokinase from genus Pyrobaculum.









Similar content being viewed by others
References
Amo T, Paje ML, Inagaki A, Ezaki S, Atomi H, Imanaka T (2000) Pyrobaculum calidifontis sp. nov., a novel hyperthermophilic archaeon that grows in atmospheric air. Archaea 1:113–121
Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T (2004) Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263–267
Baldwin RL (2007) Energetics of protein folding. J Mol Biol 371:283–301
Bibi T, Perveen S, Aziz I, Bashir Q, Rashid N, Imanaka T, Akhtar M (2016) Pcal_1127, a highly stable and efficient ribose-5-phosphate pyrophosphokinase from Pyrobaculum calidifontis. Extremophiles 20:821–830
Chohan SM, Rashid N (2013) TK1656, a thermostable l-asparaginase from Thermococcus kodakaraensis, exhibiting highest ever reported enzyme activity. J Biosci Bioeng 116:438–443
Dörr C, Zaparty M, Tjaden B, Brinkmann H, Siebers B (2003) The hexokinase of the hyperthermophile Thermoproteus tenax ATP-dependent hexokinases and ADP-dependent glucokinases, two alternatives for glucose phosphorylation in archaea. J Biol Chem 278:18744–18753
Fukui T, Atomi H, Kanai T, Matsumi R, Fujiwara S, Imanaka T (2005) Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res 15:352–363
Gharib G, Rashid N, Bashir Q, Gardner QT, Akhtar M, Imanaka T (2016) Pcal_1699, an extremely thermostable malate dehydrogenase from hyperthermophilic archaeon Pyrobaculum calidifontis. Extremophiles 20:57–67
Goward CR, Hartwell R, Atkinson T, Scawen MD (1986) The purification and characterization of glucokinase from the thermophile Bacillus stearothermophilus. Biochem J 237:415–420
Han B, Liu H, Hu X, Cai Y, Zheng D, Yuan Z (2007) Molecular characterization of a glucokinase with broad hexose specificity from Bacillus sphaericus strain C3-41. Appl Environ Microbiol 73:3581–3586
Hansen T, Schonheit P (2003) ATP-dependent glucokinase from the hyperthermophilic bacterium Thermotoga maritima represents an extremely thermophilic ROK glucokinase with high substrate specificity. FEMS Microbiol Lett 226:405–411
Hansen T, Reichstein B, Schmid R, Schonheit P (2002) The first archaeal ATP-dependent glucokinase from the hyperthermophilic crenarchaeon Aeropyrum pernix, represents a monomeric, extremely thermophilic ROK glucokinase with broad hexose specificity. J Bacteriol 184:5955–5965
Hensel R (1993) Proteins of extreme thermophiles. New Comp Biochem 26:209–221
Hsieh PC, Shenoy BC, Samols D, Phillips NFB (1996) Cloning, expression and characterization of polyphosphate glucokinase from Mycobacterium tuberculosis. J Biol Chem 271:4909–4915
Imriskova I, Arreguin-Espinosa R, Guzman S, Rodriguez-Sanoja R, Langley E, Sanchez S (2005) Biochemical characterization of the glucose kinase from Streptomyces coelicolor compared to Streptomyces peucetius var. caesius. Res Microbiol 156:361–366
Kengen SW, Tuininga JE, de Bok FA, Stams AJ, de Vos WM (1995) Purification and characterization of a novel ADP-dependent glucokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem 270:30453–30457
Koga S, Yoshioka I, Sakuraba H, Takahashi M, Sakasegawa S, Shimizu S, Ohshima T (2000) Biochemical characterization, cloning, and sequencing of ADP-dependent (AMP-forming) glucokinase from two hyperthermophilic archaea, Pyrococcus furiosus and Thermococcus litoralis. J Biochem 128:1079–1085
Labes A, Schönheit P (2003) ADP-dependent glucokinase from the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus strain 7324. Arch Microbiol 180:69–75
Lunin VV, Li Y, Schrag JD, Iannuzzi P, Cygler M, Matte A (2004) Crystal Structures of Escherichia coli ATP-dependent glucokinase and its complex with glucose. J Bacteriol 186:6915–6927
Mesak LR, Mesak FM, Dahl MK (2004) Bacillus subtilis GlcK activity requires cysteines within a motif that discriminates microbial glucokinases into two lineages. BMC Microbiol 4:1–10
Morikawa M, Izawa Y, Rashid N, Hoaki T, Imanaka T (1994) Purification and characterization of a thermostable thiol protease from a newly isolated hyperthermophilic Pyrococcus sp. Appl Environ Microbiol 60:4559–4566
Muir JM, Russell RJ, Hough DW, Danson MJ (1995) Citrate synthase from the hyperthermophilic archaeon, Pyrococcus furiosus. Protein Eng 8:583–592
Pace CN (2009) Energetics of protein hydrogen bonds. Nat Struct Mol Biol 16:681–682
Porter EV, Chassy BM, Holmlund CE (1982) Purification and kinetic characterization of a specific glucokinase from Streptococcus mutans OMZ70 cells. Biochim Biophys Acta 709:178–186
Rashid N, Morikawa M, Imanaka T (1997) Gene cloning and characterization of recombinant ribose phosphate pyrophosphokinase from a hyperthermophilic archaeon. J Biosci Bioeng 83:412–418
Rasool N, Rashid N, Iftikhar S, Akhtar M (2010) N-terminal deletion of Tk1689, a subtilisin-like serine protease from Thermococcus kodakaraensis, copes with its cytotoxicity in Escherichia coli. J Biosci Bioeng 110:381–385
Ronimus RS, Morgan HW (2004) Cloning and biochemical characterization of a novel mouse ADP-dependent glucokinase. Biochem Biophys Res Commun 315:652–658
Russell RJ, Taylor GL (1995) Engineering thermostability: lessons from thermophilic proteins. Curr Opin Biotechnol 6:370–374
Russell RJ, Ferguson JMC, Haugh DW, Danson MJ, Taylor GL (1997) The crystal structure of citrate synthase from the hyperthermophilic archaeon Pyrococcus furiosus at 1.9 Å resolution. Biochemistry 36:9983–9994
Sakuraba H, Yoshioka I, Koga S, Takahashi M, Kitahama Y, Satomura T, Kawakami R, Ohshima T (2002) ADP-dependent glucokinase/phosphofructokinase, a novel bifunctional enzyme from the hyperthermophilic archaeon Methanococcus jannaschii. J Biol Chem 277:12495–12498
Sakuraba H, Mitani Y, Goda S, Kawarabayasi Y, Ohshima T (2003) Cloning, expression, and characterization of the first archaeal ATP-dependent glucokinase from aerobic hyperthermophilic archaeon Aeropyrum pernix. J Biochem 133:219–224
Sakuraba H, Goda S, Ohshima T (2004) Unique sugar metabolism and novel enzymes of hyperthermophilic archaea. Chem Rec 3:281–287
Titgemeyer F, Reizer J, Reizer A, Saier MH Jr (1994) Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology 140:2349–2354
Tu J, Tuch BE (1996) Glucose regulates the maximal velocities of glucokinase and glucose utilization in the immature fetal rat pancreatic islet. Diabetes 45:1068–1075
Wu G, Henze K, Müller M (2001) Evolutionary relationships of the glucokinase from the amitochondriate protist, Trichomonas vaginalis. Gene 264:265–271
Acknowledgements
This work was partly supported by an NRPU Grant No. 20-2024 to NR from Higher Education Commission of Pakistan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Huang.
Rights and permissions
About this article
Cite this article
Bibi, T., Ali, M., Rashid, N. et al. Enhancement of gene expression in Escherichia coli and characterization of highly stable ATP-dependent glucokinase from Pyrobaculum calidifontis . Extremophiles 22, 247–257 (2018). https://doi.org/10.1007/s00792-017-0993-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-017-0993-4