Abstract
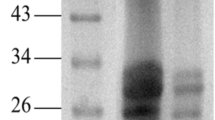
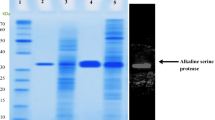
As an important class of proteases, serine proteases are required to show high activity under diverse conditions, especially at high temperatures. In the current study, two serine proteases SP348 and SP404 were analyzed by different bioinformatics tools. Both proteins are comprised of a trypsin domain and a PDZ domain, and belong to the trypsin family of proteases. The proteins were successfully expressed with Trx-tags as soluble proteins in the specialized Escherichia coli Rosetta-gami B(DE3)pLysS strain. A simple three-step purification protocol involving heat treatment, Ni–NTA purification and gel filtration was adopted to purify SP404. The molecular weight of recombinant SP404 was about 64 kDa. According to the circular dichroism spectroscopy analysis, SP404 is thermostable at 70 °C with alpha-helix, beta-sheet and random coil contents of about 8, 22 and 70 %, respectively. Our findings may broaden the range of microorganism-derived proteases and have a wide potential for industrial and fundamental studies.





Similar content being viewed by others
References
Alarico S, Empadinhas N, da Costa MS (2013) A new bacterial hydrolase specific for the compatible solutes α-d-mannopyranosyl-(1– > 2)-d-glycerate and α-d-glucopyranosyl-(1– > 2)-d-glycerate. Enzym Microb Technol 52:77–83
Argos P, Rossman MG, Grau UM, Zuber H, Frank G, Tratschin JD (1979) Thermal stability and protein structure. Biochemistry 18:5698–5703
Berezovsky IN, Chen WW, Choi PJ, Shakhnovich EI (2005) Entropic stabilization of proteins and its proteomic consequences. PLoS Comput Biol 1:e47
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Catara G, Ruggiero G, La Cara F, Digilio FA, Capasso A, Rossi M (2003) A novel extracellular subtilisin-like protease from the hyperthermophile Aeropyrum pernix K1: biochemical properties, cloning, and expression. Extremophiles 7:391–399
Chand S, Mishra P (2003) Research and application of microbial enzymes—India’s contribution. Adv Biochem Eng Biotechnol 85:95–124
Chang QL, Chen JY (1997) Separation of alpha-amylase by reversed micellar extraction. Effect of solvent type and cosolvent concentration on the transfer process. Appl Biochem Biotechnol 62:119–129
Chen XG, Stabnikova O, Tay JH, Wang JY, Tay ST (2004) Thermoactive extracellular proteases of Geobacillus caldoproteolyticus, sp. nov., from sewage sludge. Extremophiles 8:489–498
Cowan DA, Smolenski KA, Daniel RM, Morgan HW (1987) An extremely thermostable extracellular proteinase from a strain of the archaebacterium Desulfurococcus growing at 88 °C. Biochem J 247:121–133
Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay—casein as a substrate. J Vis Exp 1:1–2
Geourjon C, Deleage G (1995) SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci 11:681–684
Gupta R, Beg QK, Khan S, Chauhan B (2002) An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl Microbiol Biotechnol 60:381–395
Hashimoto Y, Yano T, Kuramitsu S, Kagamiyama H (2001) Disruption of Thermus thermophilus genes by homologous recombination using a thermostable kanamycin-resistant marker. FEBS Lett 506:231–234
Jayakumar R, Jayashree S, Annapurna B, Seshadri S (2012) Characterization of thermostable serine alkaline protease from an alkaliphilic strain Bacillus pumilus MCAS8 and its applications. Appl Biochem Biotechnol 168:1849–1866
Kleine R (1982) Properties of thermitase, a thermostable serine protease from Thermoactinomyces vulgaris. Acta Biol Med Ger 41:89–102
Koma D, Yamanaka H, Moriyoshi K, Ohmoto T, Sakai K (2007) Overexpression and characterization of thermostable serine protease in Escherichia coli encoded by the ORF TTE0824 from Thermoanaerobacter tengcongensis. Extremophiles 11:769–779
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Letunic I, Doerks T, Bork P (2015) SMART: recent updates, new developments and status in 2015. Nucleic Acids Res 43:D257–D260
Li AN, Ding AY, Chen J, Liu SA, Zhang M, Li DC (2007) Purification and characterization of two thermostable proteases from the thermophilic fungus Chaetomium thermophilum. J Microbiol Biotechnol 17:624–631
Liu JG, Yin MM, Zhu H, Lu JR, Cui ZF (2011) Purification and characterization of a hyperthermostable Mn-superoxide dismutase from Thermus thermophilus HB27. Extremophiles 15:221–226
Lu BS, Wang GL, Huang PT (1998) A comparison of amino acid composition of proteins from thermophiles and mesophiles. Wei Sheng Wu Xue Bao 38:20–25
Maehara T, Hoshino T, Nakamura A (2008) Characterization of three putative Lon proteases of Thermus thermophilus HB27 and use of their defective mutants as hosts for production of heterologous proteins. Extremophiles 12:285–296
Majeed T, Tabassum R, Orts WJ, Lee CC (2013) Expression and characterization of Coprothermobacter proteolyticus alkaline serine protease. Sci World J 2013:396156
Menzella HG (2011) Comparison of two codon optimization strategies to enhance recombinant protein production in Escherichia coli. Microb Cell Fact 10:15
Nishio Y, Nakamura Y, Kawarabayasi Y, Usuda Y, Kimura E, Sugimoto S, Matsui K, Yamagishi A, Kikuchi H, Ikeo K, Gojobori T (2003) Comparative complete genome sequence analysis of the amino acid replacements responsible for the thermostability of Corynebacterium efficiens. Genome Res 13:1572–1579
Park HJ, Reiser CO, Kondruweit S, Erdmann H, Schmid RD, Sprinzl M (1992) Purification and characterization of a NADH oxidase from the thermophile Thermus thermophilus HB8. Eur J Biochem 205:881–885
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–635
Sakamoto S, Terada I, Iijima M, Ohta T, Matsuzawa H (1995) Expression of aqualysin I (a thermophilic protease) in soluble form in Escherichia coli under a bacteriophage T7 promoter. Biosci Biotechnol Biochem 59:1438–1443
Schallmey M, Singh A, Ward OP (2004) Developments in the use of Bacillus species for industrial production. Can J Microbiol 50:1–17
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tripathi LP, Sowdhamini R (2008) Genome-wide survey of prokaryotic serine proteases: analysis of distribution and domain architectures of five serine protease families in prokaryotes. BMC Genom 9:549
Volkl P, Markiewicz P, Stetter KO, Miller JH (1994) The sequence of a subtilisin-type protease (aerolysin) from the hyperthermophilic archaeum Pyrobaculum aerophilum reveals sites important to thermostability. Protein Sci 3:1329–1340
Voorhorst WG, Eggen RI, Geerling AC, Platteeuw C, Siezen RJ, Vos WM (1996) Isolation and characterization of the hyperthermostable serine protease, pyrolysin, and its gene from the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem 271:20426–20431
Watanabe S, Muramatsu T, Ao H, Hirayama Y, Takahashi K, Tanokura M, Kuchino Y (1999) Molecular cloning of the Lon protease gene from Thermus thermophilus HB8 and characterization of its gene product. Eur J Biochem 266:811–819
Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF (1999) Protein identification and analysis tools in the ExPASy server. Methods Mol Biol 112:531–552
Zhu GP, Xu C, Teng MK, Tao LM, Zhu XY, Wu CJ, Hang J, Niu LW, Wang YZ (1999) Increasing the thermostability of d-xylose isomerase by introduction of a proline into the turn of a random coil. Protein Eng 12:635–638
Acknowledgments
This work was financially supported by the 863 Program (2015AA020925), Chinese Postdoc Fund (2014M551981), Natural Science Foundation of China (41276135 and 31172010), Program for New Century Excellent Talents in University (NCET-13-1031), Shandong Provincial Natural Science Foundation (ZR2015CL003), and Fundamental Research Funds for the Central Universities (15CX05015A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. Driessen.
Rights and permissions
About this article
Cite this article
Li, H., Sun, Y., Jiao, X. et al. Purification and characterization of thermostable serine proteases encoded by the genes ttha0099 and ttha01320 from Thermus thermophilus HB8. Extremophiles 20, 493–502 (2016). https://doi.org/10.1007/s00792-016-0839-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-016-0839-5