Abstract
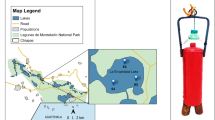
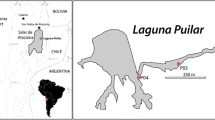
The effect of vertical physico-chemical stratification on the planktonic microbial community composition of the deep, hypersaline and heliothermal Lake Ursu (Sovata, Romania) was examined in this study. On site and laboratory measurements were performed to determine the physical and chemical variables of the lake water, and culture-based and cultivation-independent techniques were applied to identify the members of microbial communities. The surface of the lake was characterized by a low salinity water layer while the deepest region was extremely saline (up to 300 g/L salinity). Many parameters (e.g. photosynthetically active radiation, dissolved oxygen concentration, pH, redox potential) changed dramatically from 2 to 4 m below the water surface in conjunction with the increasing salinity values. The water temperature reached a maximum at this depth. At around 3 m depth, there was a water layer with high (bacterio) chlorophyll content dominated by Prosthecochloris vibrioformis, a phototrophic green sulfur bacterium. Characteristic microbial communities with various prokaryotic taxa were identified along the different environmental parameters present in the different water layers. Some of these bacteria were known to be heterotrophic and therefore may be involved in the decomposition of lake organic material (e.g. Halomonas, Idiomarina and Pseudoalteromonas) while others in the transformation of sulfur compounds (e.g. Prosthecochloris). Eukaryotic microorganisms identified by molecular methods in the lake water belonged to genera of green algae (Mantionella and Picochlorum), and were restricted mainly to the upper layers.





Similar content being viewed by others

References
Alexe M, Şerban G, Fülöp-Nagy J (2006) Lacurile sărate de la Sovata (Salt lakes from Sovata). Editura Casa Cărţii de Ştiinţă, Cluj-Napoca (In Romanian)
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amann RI, Ludwig W, Schleifer K-H (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Meth 55:541–555
Benlloch S, López-López A, Casamayor EO, Øvreås L, Goddard V, Daae FL, Smerdon G, Massana R, Joint I, Thingstad F, Pedrós-Alió C, Rodríguez-Valera F (2002) Prokaryotic genetic diversity throughout the salinity gradient of a coastal solar saltern. Environ Microbiol 4:349–360
Bertoldo C, Antranikian G (2006) The order Thermococcales. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes, vol 3. Springer, New York, pp 69–81
Bethge PO (1954) On the volumetric determination of hydrogen sulfide and soluble sulfides. Anal Chim Acta 10:113–116
Borsodi AK, Kiss RI, Cech G, Vajna B, Tóth EM, Márialigeti K (2010) Diversity and activity of cultivable aerobic planktonic bacteria of a saline lake located in Sovata, Romania. Folia Microbiol 55:461–466
Borsodi AK, Felföldi T, Máthé I, Bognár V, Knáb M, Krett G, Jurecska L, Tóth EM, Márialigeti K (2013) Phylogenetic diversity of bacterial and archaeal communities inhabiting the saline Lake Red located in Sovata, Romania. Extremophiles 17:87–98
Bulgăreanu VAC, Ionescu-Ţeculescu V, Hannich D, Demeter F (1978) Date noi privind hidrologia, limnogeologia şi hidrobotanica lacului helioterm şi pelogen Ursu (Sovata). (New data regarding the hydrology, limnogeology and hydrobotany of heliothermic and pelogenic Lake Ursu [Sovata]). Acta Botanica Horti Bucurestiensis 1977–1978:89–113 (In Romanian with English abstract)
Bulgăreanu VAC, Sitaru M, Hannich D (1985) Physico-chemical stratification and heliothermy of (karstosaline?) Lake Ursu (Sovata, Romania). Theor Appl Karstol 2:165–174
Carstens D, Köllner KE, Bürgmann H, Wehrli B, Schubert CJ (2012) Contribution of bacterial cells to lacustrine organic matter based on amino sugars and D-amino acids. Geochim Cosmochim Ac 89:159–172
Casamayor EO, Schäfter H, Bañeras L, Pedros-Alio C, Muyzer G (2000) Identification and spatio-temporal differences between microbial assemblages from two neighboring sulfurous lakes: comparison by microscopy and denaturing gradient gel electrophoresis. Appl Eviron Microbiol 66:499–508
Casamayor EO, Massana R, Benlloch S, Øvreås L, Díez B, Goddard VJ, Gasol JM, Joint I, Rodríguez-Valera F, Pedrós-Alió C (2002) Changes in archaeal, bacterial and eukaryal assemblages along a salinity gradient by comparison of genetic fingerprinting methods in a multipond solar saltern. Environ Microbiol 4:338–348
Cohen Y, Krumbein WE, Goldberg M, Shilo M (1977a) Solar Lake (Sinai). 1. Physical and chemical limnology. Limnol Oceanogr 22:597–608
Cohen Y, Krumbein WE, Shilo M (1977b) Solar Lake (Sinai). 2. Distribution of photosynthetic microorganisms and primary production. Limnol Oceanogr 22:609–620
Cohen Y, Krumbein WE, Shilo M (1977c) Solar Lake (Sinai). 3. Bacterial distribution and production. Limnol Oceanogr 22:621–634
Crognale S, Máthé I, Cardone V, Stazi SR, Ráduly B (2013) Halobacterial community analysis of Mierlei saline lake in Transylvania (Romania). Geomicrobiol J. doi:10.1080/01490451.2013.774073
Cytryn E, Minz D, Oremland RS, Cohen Y (2000) Distribution and diversity of Archaea corresponding to the limnological cycle of a hypersaline stratified lake (Solar Lake, Sinai, Egypt). Appl Environ Microbiol 66:3269–3276
Daims H, Stoecker K, Wagner M (2005) Fluorescence in situ hybridization for the detection of prokaryotes. In: Osborn AM, Smith CJ (eds) Advanced methods in molecular microbial ecology. Bios-Garland, Abingdon, pp 213–239
Demergasso C, Escudero L, Casamayor EO, Chong G, Balagué V, Pedrós-Alió C (2008) Novelty and spatio-temporal heterogeneity in the bacterial diversity of hypersaline Lake Tebenquiche (Salar de Atacama). Extremophiles 12:491–504
Díez B, Pedrós-Alió C, Marsh TL, Massana R (2001) Application of denaturing gradient gel electrophoresis (DGGE) to study the diversity of marine picoeukaryotic assemblages and comparison of DGGE with other molecular techniques. Appl Environ Microbiol 67:2942–2951
Donachie SP, Hou S, Gregory TS, Malahoff A, Alam M (2003) Idiomarina loihiensis sp. nov., a halophilic gammaproteobacterium from the Loihi submarine volcano, Hawaii. Int J Syst Evol Microbiol 53:1873–1879
Du M, Chen J, Zhang X, Li A, Li Y (2007) Characterization and resuscitation of viable but nonculturable Vibrio alginolyticus VIB283. Arch Microbiol 188:283–288
Eaton AD, Clesceri LS, Rice EW, Greenberg AE, Franson MAH (eds) (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington DC
Felföldi T, Somogyi B, Márialigeti K, Vörös L (2009) Characterization of photoautotrophic picoplankton assemblages in turbid, alkaline lakes of the Carpathian Basin (Central Europe). J Limnol 68:385–395
Gibson JAE, Swadling KM, Pitman TM, Burton HR (1997) Overwintering populations of Mesodinium rubrum (Ciliophora: Haptorida) in lakes of the Vestfold Hills, East Antarctica. Polar Biol 17:175–179
Glatz RE, Lepp PW, Ward BB, Francis CA (2006) Planktonic microbial community composition across steep physical/chemical gradients in permanently ice-covered Lake Bonney, Antarctica. Geobiology 4:53–67
Gorshkova NM, Ivanova EP, Sergeev AF, Zhukova NV, Alexeeva Y, Wright JP, Nicolau DV, Mikhailov VV, Christen R (2003) Marinobacter excellens sp. nov., isolated from sediments of the Sea of Japan. Int J Syst Evol Microbiol 53:2073–2078
Hafenbradl D, Keller M, Dirmeier R, Rachel R, Roßnagel P, Burggraf S, Huber H, Stetter KO (1996) Ferroglobus placidus gen. nov., sp. nov., A novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch Microbiol 166:308–314
Hammer UT (1986) Saline lake ecosystems of the world. Dr W. Junk Publishers, Dordrecht
Hartzell P, Reed DW (2006) The genus Archaeoglobus. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes, vol 3. Springer, New York, pp 82–100
Henley WJ, Major KM, Hironaka JL (2002) Response to salinity and heat stress in two halotolerant chlorophyte algae. J Phycol 38:757–766
Henley WJ, Hironaka JL, Guillou L, Buchheim MA, Buchheim JA, Fawley MW, Fawley KP (2004) Phylogenetic analysis of the ‘Nannochloris-like’ algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov (Trebouxiophyceae, Chlorophyta). Phycologia 43:641–652
Huss VAR, Frank C, Hartmann EC, Hirmer M, Klocoucek A, Seidel BM, Wenzeler P, Kessler E (1999) Biochemical taxonomy and molecular phylogeny of the genus Chlorella sensu lato (Chlorophyta). J Phycol 35:587–598
Imhoff JF (2003) Phylogenetic taxonomy of the family Chlorobiaceae on the basis of 16S rRNA and fmo (Fenna–Matthews–Olson protein) gene sequences. Int J Syst Evol Microbiol 53:941–951
Ionescu V, Năstăsescu M, Spiridon L, Bulgăreanu VAC (1998) The biota of Romanian saline lakes on rock salt bodies: A review. Int J Salt Lake Res 7:45–80
Ionescu-Ţeculescu V, Bulgăreanu VAC, Năstăsescu M, Vaida V, Hannich D (1982) A biological stratification pattern of heliothermic and pelogenous Lake Ursu (Sovata, Romania). Acta Botanica Horti Bucurestiensis 1981–1982:185–195
Jiang H, Dong H, Zhang G, Yu B, Chapman LR, Fields MW (2006) Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in Northwestern China. Appl Environ Microbiol 72:3832–3845
Jørgensen BB, Kuenen JG, Cohen Y (1979) Microbial transformations of sulphur compounds in a stratified lake (Solar Lake, Sinai). Limnol Oceanogr 24:799–822
Kalecsinszky S (1901) Über die ungarischen warmen und heissen Kochsalzseen als natürliche Wärme-accumulatoren, sowie über die herstellung von warmen Salzseen und Wärme-accumulatoren. Földtani Közlöny (Geologische Mitteilungen)—Zeitschrift der Ungarischen Geologischen Gesselschaft 31:409–431
Keresztes ZG, Felföldi T, Somogyi B, Székely G, Dragoş N, Márialigeti K, Bartha C, Vörös L (2012) First record of picophytoplankton diversity in Central European hypersaline lakes. Extremophiles 16:759–769
Kim KK, Jin L, Yang HC, Lee S-T (2007) Halomonas gomseomensis sp. nov., Halomonas janggokensis sp. nov., Halomonas salaria sp. nov. and Halomonas denitrificans sp. nov., moderately halophilic bacteria isolated from saline water. Int J Syst Evol Microbiol 57:675–681
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA Gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
Labrenz M, Hirsch P (2001) Physiological diversity and adaptations of aerobic bacteria from different depths of hypersaline, heliothermal, and meromictic Ekho Lake (East Antarctica). Polar Biol 24:320–327
Labrenz M, Collins MD, Lawson PA, Tindall BJ, Schumann P, Hirsch P (1999) Roseovarius tolerans gen. nov., sp. nov., a budding bacterium with variable bacteriochlorophyll-a production from hypersaline Ekho Lake. Int J Syst Bacteriol 49:137–147
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lau KWK, Ren J, Wai NLM, Lau SCL, Qian PY, Wong PK, Wu M (2006) Marinomonas ostreistagni sp. nov., isolated from a pearl-oyster culture pond in Sanya, Hainan Province, China. Int J Syst Evol Microbiol 56:2271–2275
Liu C, Wu Y, Li L, Ma Y, Shao Z (2007) Thalassospira xiamenensis sp. nov. and Thalassospira profundimaris sp. nov. Int J Syst Evol Microbiol 57:316–320
Maxim I (1929) Contribuţii la explicarea fenomenului de încălzire al apelor sărate din Transilvania. Lacurile de la Sovata. (Contributions to the elucidation of the heating processes of water in the salt lakes of Transylvania. The hot salt lakes of Sovata). Revista Muzeului Geologic-Mineralogic al Universitatea din Cluj 3:49–83 (In Romanian with German abstract)
MSZ EN 12260:2004. Water quality. Determination of nitrogen. Determination of bound nitrogen (TNb), following oxidation to nitrogen oxides. (Hungarian and European standard method)
MSZ EN 1484:1998. Water analysis. Guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC). (Hungarian and European standard method)
MSZ 260-8:1968. Wastewaters analysis. Determination of hydrogen sulphide and sulphide ion. (Hungarian standard method)
Muntean V, Paşca D, Crişan R, Kiss S, Drăgan-Bularda M (1999) Enzymological research on sediments from the Ursu and Negru salt lakes (Sovata, Mureş county). Studia Universitatis “Babeş-Bolyai”. Biologia 44:199–207
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nikolausz M, Sipos R, Révész S, Székely A, Márialigeti K (2005) Observation of bias associated with re-amplification of DNA isolated from denaturing gradient gels. FEMS Microbiol Lett 244:385–390
Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann RI, Ludwig W, Backhaus H (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol 178:5636–5643
Oren A (2002) Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J Ind Microbiol Biotechnol 28:56–63
Oren A (2006) The order Halobacteriales. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes, vol 3. Springer, New York, pp 113–164
Oren A (2008) Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst 4:2
Oren A, Naftz D, Palacios P, Wurtsbaugh WA (eds) (2009) Saline lakes around the world: unique systems with unique values (Natural Resources and Environmental Issues, Vol. XV). S.J. and Jessie E. Quinney Natural Resources Research Library, Logan
Pfennig N, Overmann J (2001) Genus I. Chlorobium. In: Boone DR, Castenholz RW, Garrity GM (eds) Bergey’s manual of systematic bacteriology, vol 1, 2nd edn. Springer, New York, pp 605–610
Poli A, Esposito E, Orlando P, Lama L, Giordano A, de Appolonia F, Nicolaus B, Gambacorta A (2007) Halomonas alkaliantarctica sp. nov., isolated from saline lake Cape Russell in Antarctica, an alkalophilic moderately halophilic, exopolysaccharide-producing bacterium. Syst Appl Microbiol 30:31–38
Polz MF, Cavanaugh CM (1998) Bias in template-to-product ratios in multitemplate PCR. Appl Environ Microbiol 64:3724–3730
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Rogozin DY, Trusova MY, Khromechek EB, Degermendzhy AG (2010) Microbial community of the chemocline of the meromictic Lake Shunet (Khakassia, Russia) during summer stratification. Microbiology 79:253–261
Sipos R, Székely AJ, Palatinszky M, Révész S, Márialigeti K, Nikolausz M (2007) Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targeting bacterial community analysis. FEMS Microbiol Ecol 60:341–350
Sorokin D (1995) Sulfitobacter pontiacus gen. nov., sp. nov.—a new heterotrophic bacterium from the Black Sea, specialized on sulfite oxidation. Microbiology 64:295–305
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tindall BJ, Rossello-Mora R, Busse H-J, Ludwig W, Kämpfer P (2010) Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol 60:249–266
Torsvik V, Goksøyr J, Daae FL (1990) High diversity in DNA of soil bacteria. Appl Environ Microbiol 56:782–787
Tsai M, Ohniwa RL, Kato Y, Takeshita SL, Ohta T, Saito S, Hayashi H, Morikawa K (2011) Staphylococcus aureus requires cardiolipin for survival under conditions of high salinity. BMC Microbiol 11:13
Vaulot D, Eikrem W, Viprey M, Moreau H (2008) The divesity of small eukaryotic phytoplankton (≤ 3 μm) in marine ecosystems. FEMS Microbiol Rev 32:795–820
von Wintzingerode F, Goebel UB, Stackebrandt E (1997) Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21:213–229
Wang J, Yang D, Zhang Y, Shen J, van der Gast C, Hahn MW, Wu Q (2011) Do patterns of bacterial diversity along salinity gradients differ from those observed for macroorganisms? PLoS ONE 6:e27597
Wetzel RG (2001) Limnology. Academic Press, San Diego
Xu XW, Wu YH, Zhou Z, Wang CS, Zhou YG, Zhang HB, Wang Y, Wu M (2007) Halomonas saccharevitans sp. nov., Halomonas arcis sp. nov. and Halomonas subterranea sp. nov., halophilic bacteria isolated from hypersaline environments of China. Int J Syst Evol Microbiol 57:1619–1624
Acknowledgments
We thank to the Research Programs Institute of Sapientia Foundation (Grant No. 209/37/2009) for supporting our research and to grant POSCCE-A2-O2.1.1-2010-2 (No. 565/09.09.2013, Code: 1734, Acronym: SILOPREP) for providing financial support for the establishment of Lake Ursu bacterial culture collection. The authors wish to thank Rita Sipos and Éva Mészáros for helpful discussions regarding the applied PCR-DGGE primers and protocols. Meteorological data were provided by Gergely Makkai from the National Meteorological Administration—Târgu Mureş (Romania). We acknowledge the staff at Balneoclimaterica SA Sovata, and we are also grateful to Toposervice SRL and to László Szakács for their help. T. F. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
Electronic supplementary material
Below is the link to the electronic supplementary material.
792_2014_633_MOESM1_ESM.tif
Supplementary Fig. 1 Selected epifluorescence microscopic images of water samples from Lake Ursu after DAPI staining. Curved cells are marked with arrows. Bar, 10 μm (TIFF 14354 kb)
Rights and permissions
About this article
Cite this article
Máthé, I., Borsodi, A.K., Tóth, E.M. et al. Vertical physico-chemical gradients with distinct microbial communities in the hypersaline and heliothermal Lake Ursu (Sovata, Romania). Extremophiles 18, 501–514 (2014). https://doi.org/10.1007/s00792-014-0633-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0633-1