Abstract
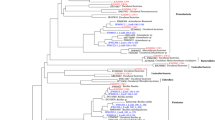
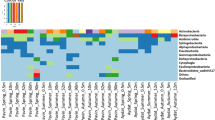
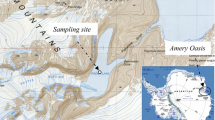
Lake Tebenquiche is one of the largest saline water bodies in the Salar de Atacama at 2,500 m above sea level in northeastern Chile. Bacteria inhabiting there have to deal with extreme changes in salinity, temperature and UV dose (i.e., high environmental dissimilarity in the physical landscape). We analyzed the bacterioplankton structure of this lake by 16S rRNA gene analyses along a spatio–temporal survey. The bacterial assemblage within the lake was quite heterogeneous both in space and time. Salinity changed both in space and time ranging between 1 and 30% (w/v), and total abundances of planktonic prokaryotes in the different sampling points within the lake ranged between two and nine times 106 cells mL−1. Community composition changed accordingly to the particular salinity of each point as depicted by genetic fingerprinting analyses (denaturing gradient gel electrophoresis), showing a high level of variation in species composition from place to place (beta-diversity). Three selected sites were analyzed in more detail by clone libraries. We observed a predominance of Bacteroidetes (about one third of the clones) and Gammaproteobacteria (another third) with respect to all the other bacterial groups. The diversity of Bacteroidetes sequences was large and showed a remarkable degree of novelty. Bacteroidetes formed at least four clusters with no cultured relatives in databases and rather distantly related to any known 16S rRNA sequence. Within this phylum, a rich and diverse presence of Salinibacter relatives was found in the saltiest part of the lake. Lake Tebenquiche included several novel microorganisms of environmental importance and appeared as a large unexplored reservoir of unknown bacteria.







Similar content being viewed by others
References
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Antón J, Oren A, Benlloch S, Rodríguez-Valera F, Amann R, Rosselló-Mora R (2002) Salinibacter ruber gen. nov., sp nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int J Syst Evol Microbiol 52:485–491
Baldauf S (2003) The deep roots of eukaryotes. Science 300:1703–1706
Benlloch S, Acinas SG, Antón J, López-López A, Luz SP, Rodríguez-Valera F (2001) Archaeal Biodiversity in Crystallizer Ponds from a Solar Saltern: Culture versus PCR. Microb Ecol 41:12–19
Benlloch S, Acinas SG, Martínez-Murcia A, Rodríguez-Valera F (1996) Description of prokaryotic biodiversity along the salinity gradient of a multipond solar saltern by direct PCR amplification of 16S rDNA. Hydrobiologia 329:19–31
Benlloch S, López-López A, Casamayor EO, Øvreas L, Goddard V, Daae FL, Smerdon G, Massana R, Joint I, Thingstad F, Pedrós-Alió C, Rodríguez-Valera F (2002) Prokaryotic genetic diversity throughout the salinity gradient of a coastal solar saltern. Environ Microbiol 4:349–360
Bowman JP, McCammon SA, Rea SM, McMeekin TA (2000) The microbial composition of three limnologically disparate hypersaline Antarctic lakes. FEMS Microbiol Lett 183:81–88
Carmona V, Pueyo JJ, Taberner C, Chong G, Thirlwall M (2000) Solute inputs in the Salar de Atacama (N. Chile). J Geochem Explor 69:449–452
Casamayor E, Muyzer G, Pedrós-Alió C (2001) Composition and temporal dynamics of planktonic archaeal assemblages from anaerobic sulfurous environments studied by 16S rDNA Denaturing Gradient Gel Electrophoresis and sequencing. Aquat Microb Ecol 25:237–246
Casamayor EO, Calderón-Paz JI, Pedrós-Alió C (2000) 5S rRNA fingerprints of marine bacteria, halophilic archaea and natural prokaryotic assemblages along a salinity gradient. FEMS Microbiol Ecol 34:113–119
Casamayor EO, Massana R, Benlloch S, Øvreas L, Díez B, Goddard VJ, Gasol JM, Joint I, Rodríguez-Valera F, Pedrós-Alió C (2002) Changes in archaeal, bacterial and eukaryal assemblages along a salinity gradient by comparison of genetic fingerprinting methods in a multipond solar saltern. Environ Microbiol 4:338–348
Cottrell MT, Kirchman DL (2000) Community composition of marine bacterioplankton determined by 16S rRNA gene clone libraries and fluorescence in situ hybridization. Appl Environ Microbiol 66:5116–5122
Demergasso C, Casamayor EO, Chong G, Galleguillos P, Escudero L, Pedrós-Alió C (2004) Distribution of prokaryotic genetic diversity in athalassohaline lakes of the Atacama Desert, Northern Chile. FEMS Microbiol Ecol 48:57–69
Dumestre JF, Casamayor EO, Massana R, Pedrós-Alió C (2002) Changes in bacterial and archaeal assemblages in an equatorial river induced by the water eutrophication of Petit Saut dam reservoir (French Guiana). Aquat Microb Ecol 26:209–221
Estrada M, Hendriksen P, Gasol JM, Casamayor EO, Pedrós-Alió C (2004) Diversity of planktonic photoautotrophic microorganisms along a salinity gradient as depicted by microscopy, flow cytometry, pigment analysis and DNA-based methods. FEMS Microbiol Ecol 49:281–293
Ferrera I, Massana R, Casamayor EO, Balagué V, Sánchez O, Pedrós-Alió C, Mas J (2004) High-diversity biofilm for the oxidation of sulfide-containing effluents. Appl Microbiol Biotechnol 64:726–734
Gasol J, del Giorgio P (2000) Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci Mar 64:197–224
Gasol J, Casamayor EO, Join I, Garde K, Gustavson K, Benlloch S, Díez B, Schauer M, Massana R, Pedrós-Alió C (2004) Control of heterotrophic prokaryotic abundance and growth rate in hypersaline planktonic environments. Aquat Microb Ecol 34:193–206
Giovannoni S, Rappé M (2000) Evolution, diversity, and molecular ecology of marine prokaryotes. In: Kirchman DL (ed) Microbial ecology of the oceans. Wiley Interscience, New York, pp 47–84
Giovannoni SJ, Britschgi TB, Moyer CL, Field KG (1990) Genetic diversity in Sargasso Sea bacterioplankton. Nature 345:60–63
Glöckner FO, Fuchs BM, Amann R (1999) Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl Environ Microbiol 65:3721–3726
Humayoun SB, Bano N, Hollibaugh JT (2003) Depth distribution of microbial diversity in Mono Lake, a meromictic soda lake in California. Appl Environ Microbiol 69:1030–1042
Jiang H, Dong H, Zhang G, Yu B, Chapman L, Fields M (2006) Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in northwestern China. Appl Environ Microbiol 72:3832–3845
Kunte H, Trüper H, Stan-Lotter H (2002) Halophilic microorganisms. In: Horneck G, Baumstark-Khan C (eds) Astrobiology, the quest for the conditions of life. Springer, Koln, pp 185–200
Lizama C, Monteoliva-Sanchez M, Prado B, Ramos-Cormenzana A, Weckesser J, Campos V (2001) Taxonomic study of extreme halophilic archaea isolated from the “Salar de Atacama”, Chile. Syst Appl Microbiol 24:464–474
Lizama C, Monteoliva-Sánchez M, Suárez-García A, Roselló-Mora R, Aguilera M, Campos V, Ramos-Cormenzana A (2002) Halorubrum tebenquichense sp. nov., a novel halophilic archaeon isolated from the Atacama Saltern, Chile. Int J Syst Evol Microbiol 52:149–155
Maidak BL, Cole JR, Lilburn TG, Parker CT, Saxman PR, Stredwick JM, Garrity GM, Li B, Olsen GJ, Pramanik S, Schmidt TM, Tiedje JM (2000) The RDP (Ribosomal Database Project) continues. Nucleic Acids Res 28:173–174
Mancinelli R, Fahlen T, Landheim R, Klovstad M (2004) Brines and evaporates: analogs for Martian life. Adv Space Res 33:1244–1246
Maturrano L, Santos F, Rosselló-Mora R, Antón J (2006) Microbial diversity in Maras salterns, a hypersaline environment in the peruvian andes. Appl Environ Microbiol 72:3887–3895
Oren A (2002) Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J Ind Microbiol Biot 28:56–63
Pedrós-Alió C (2005) Diversity of microbial communities: the case of solar salterns. In: Gunde-Cimerman N, Plemenitas A, Oren A (eds) Adaptations to life at high salt concentrations in archaea, bacteria, and eukarya. Cellular origins, life in extreme habitats and astrobiology (COLE) (Seckbach J, series editor), vol 9. Springer, Dordrecht, pp 71–90
Pedrós-Alió C (2006) Marine microbial diversity: can it be determined? Trends Microbiol 14:257–263
Pedrós-Alió C (2007) Dipping into the rare biosphere. Perspect Sci 315:192–193
Pommier T, Canback B, Riemann L, Bostrom KH, Simu K, Lundberg P, Tunlid A, Hagstrom A (2007) Global patterns of diversity and community structure in marine bacterioplankton. Mol Ecol 16:867–880
Prado B, del Moral A, Quesada E, Ríos R, Monteoliva-Sánchez M, Campos V, Ramos-Cormezana A (1991) Numerical taxonomy of moderately halophilic Gram negative rods isolated from the Salar de Atacama, Chile. Syst Appl Microbiol 14:275–281
Prado B, del Moral A, Campos V (1993) Distribution and Types of Heterotropic Halophilic Flora from Salar De Atacama, Chile. Toxicol Environ Chem 38:163–166
Prado B, Lizama C, Aguilera M, Ramos-Cormenzana A, Fuentes S, Campos V, Monteoliva-Sánchez M (2006) Chromohalobacter nigrandesensis sp. nov., a moderately halophilic, Gram-negative bacterium isolated from Lake Tebenquiche on the Atacama Saltern, Chile. Int J Syst Evol Microbiol 56:647–651
Risacher F, Alonso H (1996) Geochemistry of the Salar de Atacama. 2. Water evolution. Rev Geol Chile 23:123–134
Risacher F, Alonso H, Salazar C (1999) Geoquímica de aguas en cuencas cerradas: I, II y III Regiones—Chile (Technical Report S.I.T. No. 51). Convenio de Cooperación DGA, UCN, IRD, Santiago, Chile
Rodríguez-Valera F, Acinas S, Antón J (1999) Contribution of molecular techniques to the study of microbial diversity in hypersaline environments. In: Oren A (ed) Microbiology and Biogeochemistry of Hypersaline Environments. CRC Press, Boca Raton, pp 27–38
Sánchez O, Gasol J, Massana R, Mas J, Pedrós-Alió C (2007) Comparison of different denaturing gradient gel electrophoresis primer sets for the study of marine bacterioplankton communities. Appl Environ Microbiol 73:5962–5967
Schauer M, Massana R, Pedrós-Alió C (2000) Spatial differences in bacterioplankton composition along the Catalan coast (NW Mediterranean) assessed by molecular fingerprinting. FEMS Microbiol Ecol 33:51–59
Sorensen KB, Canfield DE, Teske AP, Oren A (2005) Community composition of a hypersaline endoevaporitic microbial mat. Appl Environ Microbiol 71:7352–7365
Staley J, Konopka A (1985) Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol 39:321–346
Valderrama MJ, Prado B, del Moral A, Ríos R, Ramos-Cormenzana A, Campos V (1991) Numerical taxonomy of moderately halophilic gram-positive cocci isolated from the Salar de Atacama (Chile). Microbiologia 7:35–41
Williams W (1996) The largest, highest and lowest lakes of the world: saline lakes. Verh Int Verein Limnol 26:61–79
Wu Q, Zwart G, Schauer M, Kamst-van Agterveld M, Hahn M (2006) Bacterioplankton community composition along a salinity gradient of sixteen high-mountain lakes located on the Tibetan Plateau, China. Appl Environ Microbiol 72:5478–5485
Yentsch C, Menzel D (1963) A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep-Sea Res 10:221–231
Zúñiga L, Campos V, Pinochet H, Prado B (1991) A limnological reconnaissance of Lake Tebenquiche, Salar de Atacama, Chile. Hydrobiologia 210:1–2
Acknowledgments
Sampling and measurements carried out in Chile were funded by grants FONDECYT 1030441 and FONDEF D99I1026. Measurements carried out in Barcelona were funded by grant “ATACAMA-2002” (CICYT, BOS2002-10258-E). Grant “BIOARSENICO” from Fundación BBVA funds current work in both laboratories. EOC was supported by the Programa Ramón y Cajal and REN2003-08333, and VB by grant GENμMAR (CTM2004-02586/MAR), both from the Spanish Ministerio de Educación y Ciencia. LEG was supported by the Centro de Investigación Científica y Tecnológica para la Minería, Antofagasta, Chile. We thank Juan José Pueyo for suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi.
Rights and permissions
About this article
Cite this article
Demergasso, C., Escudero, L., Casamayor, E.O. et al. Novelty and spatio–temporal heterogeneity in the bacterial diversity of hypersaline Lake Tebenquiche (Salar de Atacama). Extremophiles 12, 491–504 (2008). https://doi.org/10.1007/s00792-008-0153-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-008-0153-y