Abstract
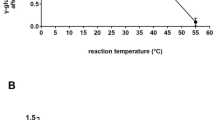
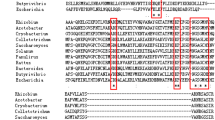
Glutamate synthase (GOGAT) is one of the two important enzymes involved in the ammonium assimilation pathway glutamine synthetase (GS)/GOGAT, which enables Hfx. mediterranei to thrive in media with low ammonium concentration or containing just nitrate as single nitrogen source. The gene coding for this enzyme, gltS, has been sequenced, analysed and compared with other GOGATs from different organisms from the three domains of life. According to its amino acid sequence, Hfx. mediterranei GOGAT displays high homology with those from other archaeal halophilic organisms and with the bacterial alpha-like subunit. Hfx. mediterranei GOGAT and GS expression was induced under conditions of ammonium restriction. The GOGAT protein was found to be a monomer with a molecular mass of 163.78 kDa, which is consistent with that estimated by gel filtration, 198 ± 30 kDa. The enzyme is highly ferredoxin dependent: activity was only observed with one of the two different 2Fe–2S ferredoxins chromatographically isolated from Hfx. mediterranei. The enzyme also displayed typical halophilic behaviour, being fully stable, and producing maximal activity, at salt concentrations from 3 to 4 M NaCl, pH 7.5 and a temperature of 50 °C.






Similar content being viewed by others
References
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Bonete MJ, Camacho ML, Cadenas E (1986) Purification and some properties of NAD-dependent glutamate dehydrogenase from Halobacterium halobium. Int J Biochem 18:785–789
Bonete MJ, Camacho ML, Cadenas E (1987) A new glutamate dehydrogenase from Halobacterium halobium with different coenzyme specificity. J Biochem 19:1149–1155
Bonete MJ, Pire C, Llorca FI, Camacho ML (1996) Glucose dehydrogenase from the halophilic archaeon Haloferax mediterranei: enzyme purification, characterization and N-terminal sequence. FEBS Lett 383:227–229
Bonete MJ, Martínez-Espinosa RM, Pire C, Zafrilla B, Richardson DJ (2008) Nitrogen metabolism in haloarchaea. Saline Syst 4:9–21
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Britton KL, Baker PJ, Fisher M, Ruzheinikov S, Gilmour DJ, Bonete MJ, Ferrer J, Pire C, Esclapez J, Rice DW (2006) Analysis of protein solvent interactions in glucose dehydrogenase from the extreme halophile Haloferax mediterranei. PNAS 103:4846–4851
Bult CJ, White O, Olsen GJ, Zhou L, Fleischmann RD, Sutton GG, Blake JA, FitzGerald LM, Clayton RA, Gocayne JD, Kerlavage AR, Dougherty BA, Tomb JF, Adams MD, Reich CI, Overbeek R, Kirkness EF, Weinstock KG, Merrick JM, Glodek A, Scott JL, Geoghagen NS, Venter JC (1996) Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1058–1073
Cabello P, Roldan MP, Moreno-Vivian C (2004) Nitrate reduction and the nitrogen cycle in archaea. Microbiology 150:3527–3546
Díaz S, Pérez-Pomares F, Pire C, Ferrer J, Bonete MJ (2006) Gene cloning, heterologous overexpression and optimized refolding of the NAD-glutamate dehydrogenase from Haloferax mediterranei. Extremophiles 10:105–115
Dyall-Smith M, Doolittle WF (1994) Construction of composite transposons for halophilic Archaea. Can J Microbiol 40:922–929
Ferrer J, Pérez-Pomares F, Bonete MJ (1996) NADP-glutamate dehydrogenase from the halophilic archaeon Haloferax mediterranei: enzyme purification, N-terminal sequence and stability. FEMS Microbiol Lett 141:59–63
Forchhammer K (2007) Glutamine signalling in bacteria. Front Biosci 12:358–370
Greene J, Henderson JW, Wikswo JP (2009) Rapid and precise determination of cellular amino acid flux rates using HPLC with automated derivatization with absorbance detection. Application note, 5990–3283N. Agilent Technologies Inc., USA
Han J, Zhang F, Hou J, Liu X, Li M, Liu H, Cai L, Zhang B, Chen Y, Zhou J, Hu S, Xiang H (2012) Complete genome sequence of the metabolically versatile halophilic archaeon Haloferax mediterranei, a poly(3-hydroxybutyrate-co-3-hydroxyvalerate) producer. J Bacteriol 194:4463–4464
Jongsareejit B, Rahman RN, Fujiwara S, Imanaka T (1997) Gene cloning, sequencing and enzymatic properties of glutamate synthase from the hyperthermophilic archaeon Pyrococcus sp. KOD1. Mol Gen Genet 254:635–642
Kameya M, Ikeda T, Nakamura M, Arai H, Ishii M, Igarashi Y (2007) A novel ferredoxin-dependent glutamate synthase from the hydrogen-oxidizing chemoautotrophic bacterium Hydrogenobacter thermophilus TK-6. J Bacteriol 189:2805–2812
Kawarabayasi Y, Sawada M, Horikawa H, Haikawa Y, Hino Y, Yamamoto S, Sekine M, Baba S, Kosugi H, Hosoyama A, Nagai Y, Sakai M, Ogura K, Otsuka R, Nakazawa H, Takamiya M, Ohfuku Y, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K, Kikuchi H (1998) Complete sequence and gene organization of the genome of the hyper-thermophilic archaebacterium Pyrococcus horikoshii OT3. DNA Res 5:55–76
Kennedy SP, Ng WV, Salzberg SL, Hood L, DasSarma S (2001) Understanding the adaptation of Halobacterium species NRC-1 to its extreme environment through computational analysis of its genome sequence. Genome Res 11:1641–1650
Klenk HP, Clayton RA, Tomb JF, White O, Nelson KE, Ketchum KA, Dodson RJ, Gwinn M, Hickey EK, Peterson JD, Richardson DL, Kerlavage AR, Graham DE, Kyrpides NC, Fleischmann RD, Quackenbush J, Lee NH, Sutton GG, Gill S, Kirkness EF, Dougherty BA, McKenney K, Adams MD, Loftus B, Peterson S, Reich CI, McNeil LK, Badger JH, Glodek A, Zhou L, Overbeek R, Gocayne JD, Weidman JF, McDonald L, Utterback T, Cotton MD, Spriggs T, Artiach P, Kaine BP, Sykes SM, Sadow PW, D'Andrea KP, Bowman C, Fujii C, Garland SA, Mason TM, Olsen GJ, Fraser CM, Smith HO, Woese CR, Venter JC (1997) Complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature 390:364–370
Lin JT, Stewart V (1997) Nitrate assimilation by bacteria. In: Poole RK (ed) Advances in microbial physiology. Academic Press, New York, pp 1–30
Lledó B, Marhuenda-Egea FC, Martínez-Espinosa RM, Bonete MJ (2005) Identification and transcriptional analysis of nitrate assimilation genes in the halophilic archaeon Haloferax mediterranei. Gene 361:80–88
Lynch EA, Langille MGI, Darling A, Wilbanks EG, Haltiner C et al (2012) Sequencing of seven haloarchaeal genomes reveals patterns of genomic flux. PLoS ONE 7(7):e41389. doi:10.1371/journal.pone.0041389
Martínez-Espinosa RM, Marhuenda-Egea FC, Bonete MJ (2001a) Purification and characterization of a possible assimilatory nitrite reductase from the halophilic archaeon Haloferax mediterranei. FEMS Microbiol Lett 196:113–118
Martínez-Espinosa RM, Marhuenda-Egea FC, Bonete MJ (2001b) Assimilatory nitrate reductase from the haloarchaeon Haloferax mediterranei: purification and characterization. FEMS Microbiol Lett 204:381–385
Martínez-Espinosa RM, Marhuenda-Egea FC, Donaire A, Bonete MJ (2003) NMR studies of a ferredoxin from Haloferax mediterranei and its physiological role in nitrate assimilatory pathway. Biochim Biophys Acta 1623:47–51
Martínez-Espinosa RM, Esclapez J, Bautista V, Bonete MJ (2006) An octameric prokaryotic glutamine synthetase from the halophilic archaeon Haloferax mediterranei. FEMS Microbiol Lett 264:110–116
Martínez-Espinosa RM, Lledó B, Marhuenda-Egea FC (2007a) The effect of ammonium on assimilatory nitrate reduction in the archaeon Haloferax mediterranei. Extremophiles 11:759–767
Martínez-Espinosa RM, Richardson DJ, Butt JN, Bonete MJ (2007b) Spectopotentiometric properties and salt-dependent thermotolerance of a [2Fe–2S] ferredoxin-involved nitrate assimilation in Haloferax mediterranei. FEMS Microbiol Lett 277:50–55
Navarro F, Chávez S, Candau P, Florencio FJ (1995) Existence of two ferredoxin-glutamate synthases in the cyanobacterium Synechocystis spl PCC 6803- Isolation and insertional inactivation of gltB and gltS genes. Plant Mol Biol 27:753–767
Nesbø CL, L’Haridon S, Stetter KO, Doolittle WF (2001) Phylogenetic analyses of two “archaeal” genes in thermotoga maritima reveal multiple transfers between archaea and bacteria. Mol Biol Evol 18:362–375
Pérez-Pomares F, Bautista V, Ferrer J, Pire C, Marhuenda-Egea FC, Bonete MJ (2003) α-Amylase activity from the halophilic archaeon Haloferax mediterranei. Extremophiles 7:299–3006
Rodriguez-Valera F, Ruiz-Berraquero F, Ramos-Cormenzana A (1980) Isolation of extremely halophilic bacteria able to grow on defined inorganic media with single carbon sources. J Gen Microbiol 119:535–538
Smith DR, Doucette-Stamm LA, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Reeve JN (1997) Complete genome sequence of Methanobacterium thermoautotrophicum deltaH: functional analysis and comparative genomics. J Bacteriol 179:7135–7155
Suzuki A, Knaff DB (2005) Glutamate synthase: structural, mechanistic and regulatory properties, and role in the amino acid metabolism. Photosynth Res 83:191–217
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–6680
van den Heuvel RH, Ferrari D, Bossi RT, Ravasio S, Curti B, Vanoni MA, Florencio FJ, Mattevi A (2002) Structural studies on the synchronization of catalytic centers in glutamate synthase. J Biol Chem 277:24579–24583
van den Heuvel RH, Svergun DI, Petoukhov MV, Coda A, Curti B, Ravasio S, Vanoni MA, Mattevi A (2003) The active conformation of glutamate synthase and its binding to ferredoxin. J Mol Biol 330:113–128
Vanoni MA, Curti B (1999) Glutamate synthase: a complex iron-sulfur flavoprotein. Cell Mol Life Sci 55:617–638
Acknowledgments
This work was supported by project BIO2008-00082 from the Spanish Ministry of Science and Innovation (MICINN), which includes funding from the European Union (“FEDER”).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Huang.
Rights and permissions
About this article
Cite this article
Pire, C., Martínez-Espinosa, R.M., Pérez-Pomares, F. et al. Ferredoxin-dependent glutamate synthase: involvement in ammonium assimilation in Haloferax mediterranei . Extremophiles 18, 147–159 (2014). https://doi.org/10.1007/s00792-013-0606-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0606-9