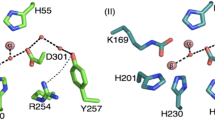
Abstract
Organophosphates (OPs) constitute the largest class of insecticides used worldwide and certain of them are potent nerve agents. Consequently, enzymes degrading OPs are of paramount interest, as they could be used as bioscavengers and biodecontaminants. Looking for a stable OPs catalyst, able to support industrial process constraints, a hyperthermophilic phosphotriesterase (PTE) (SsoPox) was isolated from the archaeon Sulfolobus solfataricus and was found to be highly thermostable. The solved 3D structure revealed that SsoPox is a noncovalent dimer, with lactonase activity against “quorum sensing signals”, and therefore could represent also a potential weapon against certain pathogens. The structural basis of the high thermostability of SsoPox has been investigated by performing a careful comparison between its structure and that of two mesophilic PTEs from Pseudomonas diminuta and Agrobacterium radiobacter. In addition, the conformational stability of SsoPox against the denaturing action of temperature and GuHCl has been determined by means of circular dichroism and fluorescence measurements. The data suggest that the two fundamental differences between SsoPox and the mesophilic counterparts are: (a) a larger number of surface salt bridges, also involved in complex networks; (b) a tighter quaternary structure due to an optimization of the interactions at the interface between the two monomers.






Similar content being viewed by others
Abbreviations
- OPs:
-
Organophosphates
- PTE:
-
Phosphotriesterase
- OPD:
-
Organophosphate-degrading
- PLL:
-
Phosphotriesterase-like lactonase
References
Afriat L, Roodveldt C, Manco G, Tawfik DS (2006) The latent promiscuity of newly identified microbial lactonases is linked to a recently diverged phosphotriesterase. Biochemistry 45:13677–13686
Aubert SD, Li Y, Raushel FM (2004) Mechanism for the hydrolysis of organophosphates by the bacterial phosphotriesterase. Biochemistry 43:5707–5715
Bruins ME, Janssen AE, Boom RM (2001) Thermozymes and their applications: a review of recent literature and patents. Appl Biochem Biotechnol 90:155–186
Chan MK, Mukund S, Kletzin A, Adams MW, Rees DC (1995) Structure of a hyperthermophilic tungstopterin enzyme, aldehyde ferredoxin oxidoreductase. Science 267:1463–1469
Cheng TC, Harvey SP, Stroup AN (1993) Purification and properties of a highly active organophosphorus acid anhydrolase from Alteromonas undina. Appl Environ Microbiol 59:3138–3140
D’Amico S, Marx JC, Gerday C, Feller G (2003) Activity–stability relationships in extremophilic enzymes. J Biol Chem 278:7891–7896
Danciulescu C, Ladenstein R, Nilsson L (2007) Dynamic arrangement of ion pairs and individual contributions to the thermal stability of the cofactor-binding domain of glutamate dehydrogenase from Thermotoga maritima. Biochemistry 46:8537–8549
De Simone G, Menchise V, Manco G, Mandrich L, Sorrentino N, Lang D, Rossi M, Pedone C (2001) The crystal structure of a hyper-thermophilic carboxylesterase from the archaeon Archaeoglobus fulgidus. J Mol Biol 314:507–518
Del Vecchio P, Graziano G, Granata V, Barone G, Mandrich L, Manco G, Rossi M (2002a) Temperature- and denaturant-induced unfolding of two thermophilic esterases. Biochemistry 41:1364–1371
Del Vecchio P, Graziano G, Granata V, Barone G, Mandrich L, Rossi M, Manco G (2002b) Denaturing action of urea and guanidine hydrochloride towards two thermophilic esterases. Biochem J 367:857–863
Del Vecchio P, Graziano G, Granata V, Farias T, Barone G, Mandrich L, Rossi M, Manco G (2004) Denaturant-induced unfolding of the acetyl-esterase from Escherichia coli. Biochemistry 43:14637–14643
DeLano WL (2002) The PyMOL molecular graphics system DeLano scientific. San Carlos, CA
Demirjian DC, Moris-Varas F, Cassidy CS (2001) Enzymes from extremophiles. Curr Opin Chem Biol 5:144–151
Dong Y-H, Wang L-H, Xu J-L, Zhang H-B, Zhang X-F, Zhang L-H (2001) Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813–817
Elias M, Dupuy J, Merone L, Lecomte C, Rossi M, Masson P, Manco G, Chabriere E (2007) Crystallization and preliminary X-ray diffraction analysis of the hyperthermophilic Sulfolobus solfataricus phosphotriesterase. Acta Crystallograph Sect F Struct Biol Cryst Commun 63:553–555
Elias M, Dupuy J, Merone L, Mandrich L, Porzio E, Moniot S, Rochu D, Lecomte C, Rossi M, Masson P, Manco G, Chabriere E (2008) Structural basis for natural lactonase and promiscuous phosphotriesterase activities. J Mol Biol 379:1017–1028
Farber GK, Petsko GA (1990) The evolution of alpha/beta barrel enzymes. Trends Biochem Sci 15:228–234
Fukuchi S, Nishikawa K (2001) Protein surface amino acid compositions distinctively differ between thermophilic and mesophilic bacteria. J Mol Biol 309:835–843
Ghanem E, Raushel FM (2005) Detoxification of organophosphate nerve agents by bacterial phosphotriesterase. Toxicol Appl Pharmacol 207:459–470
Gill SC, von Hippel PH (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem 182:319–326
Goldman A (1995) How to make my blood boil. Structure 3:1277–1279
Grimsley JK, Scholtz JM, Pace CN, Wild JR (1997) Organophosphorus hydrolase is a remarkably stable enzyme that unfolds through a homodimeric intermediate. Biochemistry 36:14366–14374
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Guillot B, Lecomte C, Cousson A, Scherf C, Jelsch C (2001) High-resolution neutron structure of nicotinamide adenine dinucleotide. Acta Crystallogr D Biol Crystallogr 57:981–989
Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, Dvir H, Ravelli RB, McCarthy A, Toker L, Silman I, Sussman JL, Tawfik DS (2004) Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol 11:412–419
Hennig M, Sterner R, Kirschner K, Jansonius JN (1997) Crystal structure at 2.0 Å resolution of phosphoribosyl anthranilate isomerase from the hyperthermophile Thermotoga maritima: possible determinants of protein stability. Biochemistry 36:6009–6016
Horne I, Qiu X, Russell RJ, Oakeshott JG (2003) The phosphotriesterase gene opdA in Agrobacterium radiobacter P230 is transposable. FEMS Microbiol Lett 222:1–8
Hou X, Maser RL, Magenheimer BS, Calvet JP (1996) A mouse kidney- and liver-expressed cDNA having homology with a prokaryotic parathion hydrolase (phosphotriesterase)-encoding gene: abnormal expression in injured and polycystic kidneys. Gene 168:157–163
Jackson C, Kim HK, Carr PD, Liu JW, Ollis DL (2005) The structure of an enzyme-product complex reveals the critical role of a terminal hydroxide nucleophile in the bacterial phosphotriesterase mechanism. Biochim Biophys Acta 1752:56–64
Jackson CJ, Foo JL, Kim HK, Carr PD, Liu JW, Salem G, Ollis DL (2008) In crystallo capture of a Michaelis complex and product-binding modes of a bacterial phosphotriesterase. J Mol Biol 375:1189–1196
Jaenicke R, Bohm G (1998) The stability of proteins in extreme environments. Curr Opin Struct Biol 8:738–748
Jones S, Thornton JM (1996) Principles of protein–protein interactions. Proc Natl Acad Sci USA 93:13–20
Karshikoff A, Ladenstein R (2001) Ion pairs and the thermotolerance of proteins from hyperthermophiles: a “traffic rule” for hot roads. Trends Biochem Sci 26:550–556
Koepke J, Scharff EI, Lucke C, Ruterjans H, Fritzsch G (2002) Atomic resolution crystal structure of squid ganglion DFPase. Acta Crystallogr D Biol Crystallogr 58:1757–1759
Ladenstein R, Antranikian G (1998) Proteins from hyperthermophiles: stability and enzymatic catalysis close to the boiling point of water. Adv Biochem Eng Biotechnol 61:37–85
Lakowicz JR (1983) Principles of fluorescence spectroscopy. Plenum Press, New York
LeJeune KE, Wild JR, Russell AJ (1998) Nerve agents degraded by enzymatic foams. Nature 395:27–28
Lo Conte L, Chothia C, Janin J (1999) The atomic structure of protein–protein recognition sites. J Mol Biol 285:2177–2198
McDonald IK, Thornton JM (1994) Satisfying hydrogen bonding potential in proteins. J Mol Biol 238:777–793
Merone L, Mandrich L, Rossi M, Manco G (2005) A thermostable phosphotriesterase from the archaeon Sulfolobus solfataricus: cloning, overexpression and properties. Extremophiles 9:297–305
Merone L, Mandrich L, Rossi M, Manco G (2008) Enzymes with phosphotriesterase and lactonase activities in Archaea. Current Chem Biol 2:237–248
Munnecke DM (1976) Enzymatic hydrolysis of organophosphate insecticides, a possible pesticide disposal method. Appl Environ Microbiol 32:7–13
Murshudov N, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53:240–255
Pichon-Pesme V, Jelsch C, Guillot B, Lecomte C (2004) A comparison between experimental and theoretical aspherical-atom scattering factors for charge–density refinement of large molecules. Acta Crystallogr A 60:204–208
Raushel FM (2002) Bacterial detoxification of organophosphate nerve agents. Curr Opin Microbiol 5:288–295
Rothschild LJ, Mancinelli RL (2001) Life in extreme environments. Nature 409:1092–1101
Seibert CM, Raushel FM (2005) Structural and catalytic diversity within the amidohydrolase superfamily. Biochemistry 44:6383–6391
Sethunathan N, Yoshida T (1973) A Flavobacterium sp. that degrades diazinon and parathion. Can J Microbiol 19:873–875
Singh BK (2009) Organophosphorus-degrading bacteria: ecology and industrial applications. Nat Rev Microbiol 7:156–164
Sterner R, Liebl W (2001) Thermophilic adaptation of proteins. Crit Rev Biochem Mol Biol 36:39–106
Suhre K, Claverie JM (2003) Genomic correlates of hyperthermostability, an update. J Biol Chem 278:17198–17202
Szilagyi A, Zavodszky P (2000) Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: results of a comprehensive survey. Structure 8:493–504
Vetriani C, Maeder DL, Tolliday N, Yip KS, Stillman TJ, Britton KL, Rice DW, Klump HH, Robb FT (1998) Protein thermostability above 100 degrees C: a key role for ionic interactions. Proc Natl Acad Sci USA 95:12300–12305
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65:1–43
Walden H, Taylor GL, Lorentzen E, Pohl E, Lilie H, Schramm A, Knura T, Stubbe K, Tjaden B, Hensel R (2004) Structure and function of a regulated archaeal triosephosphate isomerase adapted to high temperature. J Mol Biol 342:861–875
Willard L, Ranjan A, Zhang H, Monzavi H, Boyko RF, Sykes BD, Wishart DS (2003) VADAR: a web server for quantitative evaluation of protein structure quality. Nucleic Acids Res 31:3316–3319
Wong KY, Gao J (2007) The reaction mechanism of paraoxon hydrolysis by phosphotriesterase from combined QM/MM simulations. Biochemistry 46:13352–13369
Wright HT (1991) Sequence and structure determinants of the nonenzymatic deamidation of asparagine and glutamine residues in proteins. Protein Eng 4:283–294
Acknowledgments
This research was supported by grants to E.C. by Délégation Générale pour l’Armement (CO no. 010807/03-10) and by the C.N.R.S. D.R. is under contract with Bundesministerium der Verteidigung (M/SAB/1/7/A004). We also thank MIUR project “Piano Nazionale Ricerca per le Biotecnologie Avanzate Tema II, Biocatalisi”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Matsunaga.
Pompea Del Vecchio, Mikael Elias and Luigia Merone were contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Del Vecchio, P., Elias, M., Merone, L. et al. Structural determinants of the high thermal stability of SsoPox from the hyperthermophilic archaeon Sulfolobus solfataricus . Extremophiles 13, 461–470 (2009). https://doi.org/10.1007/s00792-009-0231-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-009-0231-9