Abstract
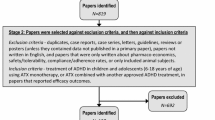
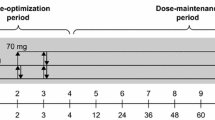
The objective of the study was to compare the efficacy and tolerability of once-daily atomoxetine (≤1.8 mg/(kg day) with those of placebo in children and adolescents (aged 6–16 years) with attention-deficit/hyperactivity disorder [ADHD (DSM-IV)]. This randomized, placebo-controlled, double-blind trial was conducted in Russia. The primary efficacy measure was baseline-to-end point changes in Attention-Deficit/Hyperactivity Disorder Rating Scale-IV-Parent Version: Investigator-Administered and Scored (ADHDRS-IV-Parent:Inv) total score. Tolerability measures included treatment-emergent signs and symptoms (TESS), laboratory values and weight. Compared with patients in the placebo group (n = 33), patients treated with atomoxetine (n = 72) with a mean final dose of 1.4 mg/kg showed significantly greater improvement in ADHDRS-IV-Parent:Inv total score (least-squares mean: atomoxetine, –15.8; placebo, –11.4; p = 0.013). The most common TESS in the atomoxetine group included anorexia [atomoxetine, n = 13 (18.1%); placebo, n = 2 (6.1%)], somnolence, n = 11 versus n = 3 (15.3% vs. 9.1%, respectively), abdominal pain n = 9 versus n = 1 (12.5% vs. 3.0%, respectively) and nausea, n = 8 versus n = 1 (11.1% vs. 3.0%, respectively). Seven patients in the atomoxetine group and two in the placebo group experienced clinically important weight loss during the study (≥7% from baseline; mean change, kg: atomoxetine, –0.6; placebo, 0.1; p = 0.032). Atomoxetine is efficacious in improving ADHD symptoms in children and adolescents. Atomoxetine treatment may be associated with a numerically higher incidence of anorexia, somnolence, abdominal pain and nausea, as well as statistically greater losses in body weight.


Similar content being viewed by others
References
Altschuler RA, Maschkovsky MD, Roshchina LF (1973) Sydnocarb is a new stimulant of the central nervous system. Farmacol Toxicol 36:18–23 (in Russian)
Barkley RA, Murrary MB, Edelbrock CS, Robbins K (1990) Side effects of methylphenidate in children with attention-deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics 86:184–192
Bashkatova V, Mathieu-Kia AM, Durand C, Penit-Soria J (2002) Neurochemical changes and neurotoxic effects of an acute treatment with sydnocarb, a novel psychostimulant: comparison with D-amphetamine. Ann N Y Acad Sci 965:180–192
Borinskaya SA, Kozhekbaeva ZhM, Gorbunova EV et al (2004) Analysis of the DRD4 gene polymorphism in populations of Russia and neighboring countries. Russ J Genet 40:679–683 (in Russian)
Conners CK, Sitarenios G, Parker JD, Epstein JN (1998) The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26:257–268
Crichton A (1798) An Inquiry into the nature and origin of mental derangement: comprehending a concise system of physiology and pathology of the human mind and history of the passions and their effects. T. Cadell Jr. and W Davies, London (Reprinted by AMS Press, New York, 1976)
DuPaul GJ, Power TJ, Anastopoulos AD, Reid R (1998) ADHD rating scale-IV: checklists, norms, and clinical interpretations. The Guilford Press, New York, NY
Faraone SV (2003) Understanding the effect size of ADHD medication: implications for clinical care. Medscape Psych Ment Health 28:2. www.medscape.com/viewarticle/461543
Faraone SV, Sergeant J, Gillberg C, Biederman J (2003) The worldwide prevalence of ADHD: is it an American condition? World Psychiatry 2:104–113
Guy W (1976) ECDEU assessment manual for psychopharmacology, revised. US Department of Health, Education, and Welfare, Bethesda, MD
Ireland W (1877) On idiocy and imbecility. JSA Churchill, London
Kaufman J, Birmaher B, Brent D et al (1997) Schedule for affective disorders and schizophrenia for school-age children present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 136:980–988
Kelsey DK, Sumner CR, Casat CD et al (2004) Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics 114:e1–e8
Kornilova TV, Grigorenko EL, Smirnov SD (2004) Teenagers of risk groups. Piter Publishers, Moscow-Saint Petersburg, pp 161–333
Krasov VA (1988) Sydnocarb treatment of young schoolchildren with hyperdynamic syndrome. Zh Nevropatol Psikhiatr Im S S Korsakova 88:97–101 (in Russian)
Kratochvil CJ, Wilens TE, Greenhill LL et al (2006) Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 45:919–927
Michelson D, Faries D, Wernike J et al (2001) Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose–response study. Pediatrics 108:E83
Michelson D, Allen AJ, Busner J et al (2002) Once-daily atomoxetine treatment for children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 159:1896–1901
Newcorn J, Allen AJ, Sutton V et al (2003) Characteristics of placebo responders in pediatric ADHD clinical trials. Paper presented at the 50th annual meeting of the American academy of child and adolescent psychiatry. Miami, FL
Newcorn HJ, Spencer TJ, Biederman J, Milton DR, Michelson D (2005) Atomoxetine treatment in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry 44:240–248
Newcorn HJ, Kratochwill CJ, Allen AJ et al (2008) Atomoxetine and osmotically released methylphenidate for the treatment of attention-deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry 165:721–730
Palmer ED, Finger S (2001) An early description of ADHD (inattentive subtype): Dr Alexander Crichton and “mental restlessness” (1798). Child Psychol Psychiatry Review 6:66–73
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and meta-regression analysis. Am J Psychiatry 164:942–948
Still GF (1902) The Culostian lectures on some abnormal psychical conditions in children. Lancet i1008–i1012
Tredgold AF (1908) Mental deficiency (amentia). Tindall and Cox, Bailliere
US Department of Health and Human Services (1995) COSTART: coding symbols of thesaurus of adverse reaction terms, 5th edn. US Food and Drug Administration, Rockville, MD
Uzbekov MG, Misionzhnik EY (2003) Changes in urinary monoamine excretion in hyperkinetic children. Hum Psychopharmacol 18:493–497
Weiss M, Tannock R, Kratochvil C et al (2005) A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry 44:647–655
Zavadenko N, Suvorinova N (1999) ADHD: study of motor control, attention, memory and experience with nootropics. In: Evrard Ph, Richelme Ch, Tardieu M (eds) Proceedings of the 3rd European paediatric neurology (EPNS) congress, Bologna, Monduzzi Editore, pp 81–85
Zavadenko NN, TIu Uspenskaia, NIu Suvorinova (1997) The diagnosis and treatment of the attention-deficit syndrome in children. Zh Nevropatol Psikhiatr Im S S Korsakova 97(1):57–61
Zavadenko N, Petrukhin A, Suvorinova N, Grigorieva N (1998) ADHD: study of prevalence, aetiology and efficiency of nootropics therapy. In: Velickovic Perat M (ed) New developments in child neurology. Monduzzi Editore, Bologna, pp 675–679
Acknowledgment
The authors thank Svetlana Dominguez for helping to prepare this paper.
Financial disclosure
Drs. Martenyi and Jarkova are employees and stockholders of Eli Lilly and Company. Dr. Zavadenko is a member of the Lilly ADHD advisory board. The rest of the authors do not have any financial disclosures to report. This study was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical Trials Registry: NCT00386581, http://www.clinicaltrials.gov/.
Rights and permissions
About this article
Cite this article
Martenyi, F., Zavadenko, N.N., Jarkova, N.B. et al. Atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: a 6-week, randomized, placebo-controlled, double-blind trial in Russia. Eur Child Adolesc Psychiatry 19, 57–66 (2010). https://doi.org/10.1007/s00787-009-0042-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-009-0042-7