Abstract
Introduction
This study investigated the presence of different Gram-negative bacterial species and the levels of endotoxins found in primary endodontic infection (PEI), determining their stimulation ability against macrophages through the levels of interleukin (IL)-1, IL-6, IL-10, and tumor necrosis factor alpha (TNF-α), and evaluated their relationship with clinical and radiographic findings.
Material and methods
Samples were taken from 21 root canals with primary endodontic infection with apical periodontitis (PEIAP). Molecular techniques were used for bacterial detection. Limulus amebocyte lysate assay was used to measure endotoxins. Pro-inflammatory cytokines were measured by ELISA assay.
Results
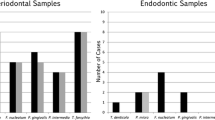
All samples were positive for bacterial DNA (21/21). Prevotella nigrescens (57.2 %) was the most frequent species. Higher levels of endotoxins were found in teeth with pain on palpation and exudation (all p < 0.05). Positive correlations were found between endotoxins and the levels of TNF-α and IL-1β, whereas a negative correlation was found between endotoxin and the amount of IL-10 (p < 0.05). Endotoxin levels were found to be a risk factor for exudation and increased the number of Gram-negative bacterial species for the presence of a larger area of bone destruction (all p < 0.05).
Conclusion
A wide variety of Gram-negative bacterial species are involved in primary endodontic infection, with participation of different Treponema species. Thus, the levels of endotoxins and the number of Gram-negative bacteria species present in root canals were considered risk factors for the severity of endodontic infection.
Clinical relevance
The present study revealed that Gram-negative bacterial species and endotoxins play an important role in the development of signs/symptoms and the severity of bone destruction, this knowledge is essential for the establishment of an effective therapy.
Similar content being viewed by others
References
Siqueira JF Jr, Rocas IN (2013) Microbiology and treatment of acute apical abscesses. Clin Microbiol Rev 26:255–273. doi:10.1128/CMR.00082-12
Martinho FC, Chiesa WM, Leite FR, Cirelli JA, Gomes BP (2010) Antigenic activity of bacterial endodontic contents from primary root canal infection with periapical lesions against macrophage in the release of interleukin-1beta and tumor necrosis factor alpha. J Endod 36:1467–1474. doi:10.1016/j.joen.2010.06.012
Montagner F, Jacinto RC, Signoretti FG, Gomes BP (2010) Treponema species detected in infected root canals and acute apical abscess exudates. J Endod 36:1796–1799. doi:10.1016/j.joen.2010.08.008
Rocas IN, Siqueira JF Jr (2010) Identification of bacteria enduring endodontic treatment procedures by a combined reverse transcriptase-polymerase chain reaction and reverse-capture checkerboard approach. J Endod 36:45–52. doi:10.1016/j.joen.2009.10.022
Rocas IN, Siqueira JF Jr, Andrade AF, Uzeda M (2003) Oral treponemes in primary root canal infections as detected by nested PCR. Int Endod J 36:20–26
Gomes BP, Lilley JD, Drucker DB (1996) Associations of endodontic symptoms and signs with particular combinations of specific bacteria. Int Endod J 29:69–75
Siqueira JF Jr, Rocas IN (2003) PCR-based identification of Treponema maltophilum, T amylovorum, T medium, and T lecithinolyticum in primary root canal infections. Arch Oral Biol 48:495–502
Metzger Z (2000) Macrophages in periapical lesions. Endod Dent Traumatol 16:1–8
Beutler B, Cerami A (1989) The biology of cachectin/TNF—a primary mediator of the host response. Annu Rev Immunol 7:625–655. doi:10.1146/annurev.iy.07.040189.003205
Keller R, Fischer W, Keist R, Bassetti S (1992) Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun 60:3664–3672
Garcia de Aquino S, Manzolli Leite FR, Stach-Machado DR, Francisco da Silva JA, Spolidorio LC, Rossa C Jr (2009) Signaling pathways associated with the expression of inflammatory mediators activated during the course of two models of experimental periodontitis. Life Sci 84:745–754. doi:10.1016/j.lfs.2009.03.001
Sousa EL, Martinho FC, Nascimento GG, Leite FR, Gomes BP (2014) Quantification of endotoxins in infected root canals and acute apical abscess exudates: monitoring the effectiveness of root canal procedures in the reduction of endotoxins. J Endod 40(2):177–181. doi:10.1016/j.joen.2013.10.008
Seymour GJ, Gemmell E (2001) Cytokines in periodontal disease: where to from here? Acta Odontol Scand 59:167–173
Martinho FC, Chiesa WM, Leite FR, Cirelli JA, Gomes BP (2012) Correlation between clinical/radiographic features and inflammatory cytokine networks produced by macrophages stimulated with endodontic content. J Endod 38:740–745. doi:10.1016/j.joen.2012.02.021
Boyce BF, Li P, Yao Z, Zhang Q, Badell IR, Schwarz EM, O’Keefe RJ, Xing L (2005) TNF-alpha and pathologic bone resorption. Keio J Med 54:127–131
Huang GT, Do M, Wingard M, Park JS, Chugal N (2001) Effect of interleukin-6 deficiency on the formation of periapical lesions after pulp exposure in mice. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92:83–88. doi:10.1067/moe.2001.115025
Tinsley JH, South S, Chiasson VL, Mitchell BM (2010) Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol 298:R713–R719. doi:10.1152/ajpregu.00712.2009
Leon LR, Kozak W, Kluger MJ (1998) Role of IL-10 in inflammation. Studies using cytokine knockout mice. Ann N Y Acad Sci 856:69–75
Gomes BP, Martinho FC, Vianna ME (2009) Comparison of 2.5 % sodium hypochlorite and 2 % chlorhexidine gel on oral bacterial lipopolysaccharide reduction from primarily infected root canals. J Endod 35:1350–1353. doi:10.1016/j.joen.2009.06.011
Rocas IN, Siqueira JF Jr, Debelian GJ (2011) Analysis of symptomatic and asymptomatic primary root canal infections in adult Norwegian patients. J Endod 37:1206–1212. doi:10.1016/j.joen.2011.05.026
Dixon DR, Darveau RP (2005) Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dent Res 84:584–595
Schein B, Schilder H (1975) Endotoxin content in endodontically involved teeth. J Endod 1:19–21. doi:10.1016/S0099-2399(75)80244-5
Hong CY, Lin SK, Kok SH, Cheng SJ, Lee MS, Wang TM, Chen CS, Lin LD, Wang JS (2004) The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J Oral Pathol Med 33:162–169
Chung YH, Chang EJ, Kim SJ, Kim HH, Kim HM, Lee SB, Ko JS (2006) Lipopolysaccharide from Prevotella nigrescens stimulates osteoclastogenesis in cocultures of bone marrow mononuclear cells and primary osteoblasts. J Periodontal Res 41:288–296. doi:10.1111/j.1600-0765.2006.00876.x
Baumgartner JC, Khemaleelakul SU, Xia T (2003) Identification of spirochetes (treponemes) in endodontic infections. J Endod 29:794–797. doi:10.1097/00004770-200312000-00002
Fenno JC, McBride BC (1998) Virulence factors of oral treponemes. Anaerobe 4:1–17. doi:10.1006/anae.1997.0131
Chan EC, McLaughlin R (2000) Taxonomy and virulence of oral spirochetes. Oral Microbiol Immunol 15:1–9
Asai Y, Hashimoto M, Ogawa T (2003) Treponemal glycoconjugate inhibits Toll-like receptor ligand-induced cell activation by blocking LPS-binding protein and CD14 functions. Eur J Immunol 33:3196–3204. doi:10.1002/eji.200324219
Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL (2001) Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol 166:2444–2450
Opitz B, Schroder NW, Spreitzer I, Michelsen KS, Kirschning CJ, Hallatschek W, Zahringer U, Hartung T, Gobel UB, Schumann RR (2001) Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappaB translocation. J Biol Chem 276:22041–22047. doi:10.1074/jbc.M010481200
Martinho FC, Leite FRM, Chiesa WMM, Nascimento GG, Feres M, Gomes BPFA (2014) Signaling pathways activation by primary endodontic infectious contents and production of inflammatory mediators. J Endod. doi:10.1016/j.joen.2013.10.022
Jiang J, Zuo J, Hurst IR, Holliday LS (2003) The synergistic effect of peptidoglycan and lipopolysaccharide on osteoclast formation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 96:738–743. doi:10.1016/S107921040300502X
Takahashi K (1998) Microbiological, pathological, inflammatory, immunological and molecular biological aspects of periradicular disease. Int Endod J 31:311–325
Acknowledgments
This work was supported by the Brazilian agencies FAPESP (08/57551-0; 08/56425; 2012/19536-5) and CNPq (3470820/2006-3; 471631/2008-6; 302575/2009-0; 150557/2011-6).
Conflict of interest
The authors deny any conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinho, F.C., Leite, F.R.M., Nascimento, G.G. et al. Clinical investigation of bacterial species and endotoxin in endodontic infection and evaluation of root canal content activity against macrophages by cytokine production. Clin Oral Invest 18, 2095–2102 (2014). https://doi.org/10.1007/s00784-014-1198-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1198-1