Abstract
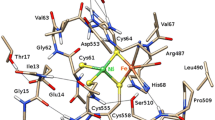
The sulfhydrogenase complex of Pyrococcus furiosus is an αβγδ heterotetramer with both hydrogenase activity (borne by the αδ subunits) and sulfur reductase activity (carried by the βγ subunits). The β-subunit contains at least two [4Fe-4S] cubanes and the γ-subunit contains one [2Fe-2S] cluster and one FAD molecule. The δ-subunit contains three [4Fe-4S] cubanes and the α-subunit carries the NiFe dinuclear center. Only three Fe/S signals are observed in EPR-monitored reduction by dithionite, NADPH, or internal substrate upon heating. All other clusters presumably have reduction potentials well below that of the H+/H2 couple. Heat-induced reduction by internal substrate allows, for the first time, EPR monitoring of the NiFe center in a hyperthermophilic hydrogenase, which passes through a number of states, some of which are similar to states previously defined for mesophilic hydrogenases. The complexity of the observed transitions reflects a combination of temperature-dependent activation and temperature-dependent reduction potentials.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 10 December 1998 / Accepted: 16 February 1999
Rights and permissions
About this article
Cite this article
Silva, P., de Castro, B. & Hagen, W. On the prosthetic groups of the NiFe sulfhydrogenase from Pyrococcus furiosus: topology, structure, and temperature-dependent redox chemistry. JBIC 4, 284–291 (1999). https://doi.org/10.1007/s007750050314
Issue Date:
DOI: https://doi.org/10.1007/s007750050314