Abstract
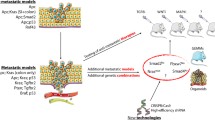
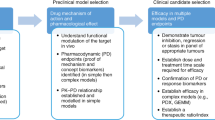
Animal models have been critical in the study of the molecular mechanisms of cancer and in the development of new antitumor agents; nevertheless, there is still much room for improvement. The relevance of each particular model depends on how close it replicates the histology, physiological effects, biochemical pathways and metastatic pattern observed in the same human tumor type. Metastases are especially important because they are the main determinants of the clinical course of the disease and patient survival, and are the target of systemic therapy. The generation of clinically relevant models using the mouse requires their humanization, since differences exist in transformation and oncogenesis between human and mouse. Although genetically modified (GM) mice have been instrumental in understanding the molecular mechanisms involved in tumor initiation, they have been less successful in replicating advanced cancer. Moreover, a particular genetic alteration frequently leads to different tumor types in human and mouse and to lower metastastatic rates in GM mice than in humans. These findings question the capacity of current GM mouse carcinoma models to predict clinical response to therapy. On the other hand, orthotopic (ORT) xenografts of human tumors, or tumor cell lines, in nude mice reproduced the histology and metastatic pattern of most human tumors at advanced stage. Usingex vivo genetic manipulation of human tumor cells, ORT models can be used to molecularly dissect the metastatic process and to evaluatein vivo tumor response to therapy, using non-invasive procedures. Nevertheless, this approach is not useful in the study of the initial stages of tumorigenesis or the contribution of the immune system in this process. Despite ORT models are more promising than the most commonly used subcutaneous xenografts in preclinical drug development, their capacity to predict clinical response to antitumor agents remains to be studied. Humanizing mouse models of cancer will most likely require the combined use of currently available methodologies.
Similar content being viewed by others
References
Van Dyke T, Jacks T. Cancer modelling in the modern era: progress and challenges. Cell. 2002;108:135–44.
Grever M, Chaner BA. The National Cancer Institute: Cancer drug discovery and development program. In: (DeVita VT, Jr., Hellman S, Rosenberg SA, eds.). Cancer principles & practice of oncology. (5th ed). Philadelphia (PA): Lippincott-Raven; 1997.
DeVita Jr VT, Hellman S, Rosenberg SA (eds.). Cancer: Principles and Practice of Oncology. 7th. Ed. Lippincott Williams & Wilkins. 2005; p. 3120.
Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20.
Weber GF (ed.): Cancer Therapy: Molecular Targets in Tumor-Host Interactions. Horizon Bioscience. Editor University of Cincinnati Medical Center, Cincinnati, OH, USA. 2005; p. 398.
Hanahan, D., Weinberg RA. The hall-marks of cancer. Cell. 2000;100:57–70.
McClatchey AI. Modeling metastasis in the mouse. Oncogene. 1999;18(38):5334–9.
Nowell PC. The clonal evolution of tumor cell populations. Science. 1976,194(4260): 23–8.
Bernards R Weinberg RA. A progression puzzle. Nature. 2002;418:823.
Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003;33(1):49–54.
Hunter K. Host genetics influence tumour metastasis. Nat Rev Cancer. 2006;6(2):141–6.
Khanna C, Hunter K. Modeling metastasisin vivo. Carcinogenesis. 2005;26(3):513–23.
Slack NH, Bross ID. The influence of site of metastasis on tumour growth and response to chemotherapy. Br J Cancer. 1975;32(1):78–86.
Fidler IJ. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev. 1991;10(3):229–43.
González FJ, Kimura S. Understanding the role of xenobiotic-metabolism in chemical carcinogenesis using gene knockout mice. Mutat Res. 2001;477(1–2):79–87.
Mouse Genome Sequence Consortium, Initial sequencing and comparative analysis of the mouse genome. Nature. 2002; 420(6915):520–62.
Rangarajan A, Weinberg RA. Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2005;3(12):952–9.
Jacks T. Tumor suppressor gene mutations in mice. Annu Rev Genet. 1996;30: 603–36.
Wagner KU. Models of breast cancer: quo vadis, animal modeling? Breast Cancer Res 2004;6(1):31–8.
Cardiff RD. Validity of mouse mammary tumour models for human breast cancer: comparative pathology. Microsc Res Tech. 2001;52(2):224–30.
Nandi S, Guzmán RC, Yang J. Hormones and mammary carcinogenesis in mice, rats, and humans: a unifying hypothesis. Proc Natl Acad Sci USA. 1995;92(9):5650–7.
Grisham JW. Interspecies comparison of liver carcinogenesis: implications for cancer risk assessment. Carcinogenesis. 1997; 18(1):59–81.
Rosol TJ, Tannehill-Gregg SH, LeRoy BE, Mandl S, Contag CH. Animal models of bone metastasis. Cancer. 2003;97(Suppl 3):S748–57.
Clarke R. Animal models of breast cancer: their diversity and role in biomedical research. Breast Cancer Res Treat. 1996; 39(1):1–6.
Hoffman R. Green fluorescent protein imaging of tumour growth, metastasis, and angiogenesis in mouse models. Lancet Oncol. 2002;3(9):546–56.
Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17(4):343–59.
Kim JB, O'Hare MJ, Stein R. Models of breast cancer: is merging human and animal models the future? Breast Cancer Res. 2004;6(1):22–30.
Heijstek MW, Kranenburg O, Borel Rinkes IH. Mouse models of colorectal cancer and liver metastases. Dig Surg. 2005;22(1–2):16–25.
Balmain A. Cancer as a complex genetic trait: tumor susceptibility in humans and mouse models. Cell. 2002;108:145–52.
Kamb A. What's wrong with our cancer models? Nat Rev Drug Discov. 2005;4(2): 161–5.
Staquel MJ, Byar DP, Green SB, Rozencweig M. Clinical predictivity of transplantable tumor systems in the selection of new drugs for solid tumors: rationale for a three-stage strategy. Cancer Treat Rep. 1983;67(9):753–65.
Gura T. Systems for identifying new drugs are often faulty. Science. 1997;273: 1041–2.
Venditti JM, Wesley RA, Plowman J. Current NCl preclinical antitumor screeningin vivo: results of tumor panel screening, 1976–1982, and future directions. Adv Pharmacol Chemother. 1984;20:1–20.
Wilmanns C, Fan D, O'Brian CA, Bucana CD, Fidler IJ. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxoribicin and 5-fluorouracil. Int J Cancer. 1992;52:98–104.
Wilmanns C, Fan D, O'Brian CA, et al. Modulation of doxorubicin sensitivity and P-glycoprotein expression in human colon carcinoma cells by ectopic and orthotopic environments in nude mice. Int J Oncol. 1993;3:412–22.
Dong Z, Radinsky R, Fan D, Tsan R, Bucana CD, Wilmanns C, Fidler IJ. Organspecific modulation of mdr gene expression and drug resistance in murine colon cancer cells. J Natl Cancer Inst. 1994;86: 913–20.
Pratesi G, Manzotti C, Tortoreto M, Audisio RA, Zunino F Differential efficacy of flavone acetic against liver versus lung metastases in a human tumour xenograft. Br J Cancer. 1991;63(1):71–4.
Staroselsky AN, Fan D, O'Brian CA, Bucana CD, Gupta KP, Fidler IJ. Site-dependent differences in response to the UV-2237 murine fibrosarcoma to systemic therapy with adriamycin. Cancer Res. 1990;50:7775–80.
Smith KA, Begg AC, Denekamp J. Differences in chemosensitivity between subcutaneous and pulmonary tumours. Eur J Cancer Clin Oncol. 1985;21(2):249–56.
Sikder H, Huso DL, Zhang H, et al. Disruption of Id1 reveals major differences in angiogenesis between transplanted and autochthonous tumors. Cancer Cell. 2003;4(4):291–9.
Alani RM, Silverthorn CF, Orosz K. Tumor angiogensis in mice and men. Cancer Biol Ther. 2004;5(6):498–500.
Johnson JI, Decker S, Zaharevitz D, et al. Relationships between drug activity in NCl prelinicalin vitro andin vivo models and early clinical trials. Br J Cancer 2001;84(10):1424–51.
Farre L, Casanova I, Guerrero S, Trias M, Capella G, Mangues R. Heterotopic implantation alters the regulation of apoptosis and the cell cycle and generates a new metastatic site in a human pancreatic tumor xenograft model. FASEB J. 2002;16 (9):975–82.
Rosenberg MP, Bortner D. Why transgenic and knockout animal models should be used (for drug efficacy studies in cancer). Cancer Metastasis Rev. 1998–99;17 (5):295–9.
Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cacer. 2002;2(5):331–41.
Clarke AR. Manipulating the germline: its impact on the study of carcinogenesis Carcinogenesis. 2000;21:435–41.
Alexander J. Use of transgenic mice in identifying chemopreventive agents. Toxicol Lett. 2000;112–113:507–12.
Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:155–64.
Berns A. Cancer. Improved mouse models. Nature. 2001;410:1045–4.
Adams JM, Cory S. Transgenic models of tumor development. Science. 1991;254: 1161–7.
Clarke AR. Manipulating the germline: its impact on the study of carcinogenesis. Carcinogenesis. 2000;21:435–41.
Hakem Rr, Mak TW. Animal models of tumor suppressor genes. Ann Rev Genet. 2001;35:209–41.
Tuveson DA, Jacks T. Technologically advanced cancer modeling in mice. Curr Opin Genet Dev. 2002;12(1):105–10.
Jonkers J, Berns A. Conditional mouse models of sporadic cancer. Nat Rev Cancer. 2002;2:251–65.
Herzig M, Christofori G. Recent advances in cancer research: mouse models of tumor igenesis. Biochim Biophys Acta. 2002;1602(2):97–113.
Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–6.
Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2002;2:743–55.
Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256(5057):668–70.
Shibata H, Toyama K, Shioya H, et al. Rapid colorectal adenoma formation initiated by conditional, targeting of the Apc gene. Science. 1997;278(5335):120–5.
Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11(1):63–70.
Aguirre AJ, Bardeesy N, Sinha M, López L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17(24):3112–26.
Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001;28(2):155–9.
Druker BJ. Imatinib as a paradigm of targeted therapies. Adv Cancer Res. 2004;91: 1–30.
Griffin JD. FLT3 tyrosine kinase as a target in acute leukemias. Hematol J. 2004;5 Suppl 3:S188–90.
Weisberg E, Griffin JD. Resistance to imatinib (Glivec): update on clinical mechanisms. Drug Resist Updat. 2003;6(5):231–8.
Knudson AG Jr. Overview: genes that predispose to cancer. Mutat Res. 1991;247 (2):185–90.
Bankert RB, Egilmez NK, Hess SD. Human-SCID mouse chimeric models for the evaluation of anti-cancer therapies. Trends Immunol. 2001;22(7):386–93.
De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003; 200(4):429–47.
Eccles SA, Fox G, Court W, Sandle J, Dean CJ. Preclinical models for the evaluation of targeted therapies of metastatic disease. Cell Biophys. 1994;24:279–91.
Hoffman RM. Orthotopic is orthodox: why are orthotopic-transplant metastatic models different from all other models? J Cell Biochem. 1994;56(1):1–3.
Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998–99;17(3):279–84.
Bibby MC. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer. 2004;40(6):852–7.
Radinsky R. Modulation of tumor cell gene expression and phenotype by the organ-specific metastatic environment. Cancer Metastasis Rev. 1995;14(4):523–58.
Pocard M, Tsukui H, Salmon RJ, Dutrillaux B, Poupon MF. Efficiency of orthotopic xenograft models for human colon cancers. In Vivo. 1996;10(5):463–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by an unrestricted educational grant from AstraZeneca.
Rights and permissions
About this article
Cite this article
Céspedes, M.V., Casanova, I., Parreño, M. et al. Mouse models in oncogenesis and cancer therapy. Clin Transl Oncol 8, 318–329 (2006). https://doi.org/10.1007/s12094-006-0177-7
Issue Date:
DOI: https://doi.org/10.1007/s12094-006-0177-7