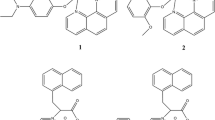
Abstract
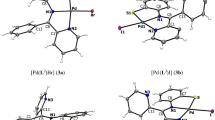
A series of oxovanadium complexes with mixed ligands, a tridentate ONO-donor Schiff base ligand [viz., salicylidene anthranilic acid (SAA)], and a bidentate NN ligand [viz., 2,2′-bipyridine (bpy), 1,10-phenanthroline (phen), dipyrido[3,2-d:2′,3′-f]quinoxaline (dpq), dipyrido[3,2-a:2′,3′-c]phenazine (dppz), or 7-methyldipyrido[3,2-a:2′,3′-c]phenazine (dppm)], have been synthesized and characterized by elemental analysis, electrospray ionization mass spectrometry, UV–vis spectroscopy, Fourier transform IR spectroscopy, EPR spectroscopy, and X-ray crystallography. Crystal structures of both complexes, [VIVO(SAA)(bpy)]·0.25bpy and [VIVO(SAA)(phen)]·0.33H2O, reveal that oxovanadium(IV) is coordinated with one nitrogen and two oxygen atoms from the Schiff base and two nitrogen atoms from the bidentate planar ligands, in a distorted octahedral geometry (VO3N3). The oxidation state of V(IV) with d 1 configuration was confirmed by EPR spectroscopy. The speciation of VO–SAA–bpy in aqueous solution was investigated by potentiomtreic pH titrations, and the results revealed that the main species are two ternary complexes at a pH range of 7.0–7.4, and one is the isolated crystalline complex. The complexes have been found to be potent inhibitors against human protein tyrosine phosphatase 1B (PTP1B) (IC50 approximately 30–61 nM), T-cell protein tyrosine phosphatase (TCPTP), and Src homology phosphatase 1 (SHP-1) in vitro. Interestingly, the [VIVO(SAA)(bpy)] complex selectively inhibits PTP1B over the other two phosphatases (approximate ninefold selectivity against SHP-1 and about twofold selectivity against TCPTP). Kinetics assays suggest that the complexes inhibit PTP1B in a competitive and reversible manner. These suggest that the complexes may be promising candidates as novel antidiabetic agents.
Graphical Abstract










Similar content being viewed by others
Abbreviations
- BMOV:
-
Bis(maltolato)oxovanadium
- bpy:
-
2,2′-Bipyridine
- dppm:
-
7-Methyldipyrido[3,2-a:2′,3′-c]phenazine
- DFT:
-
Density functional theory
- DMSO:
-
Dimethyl sulfoxide
- dppz:
-
Dipyrido[3,2-a:2′,3′-c]phenazine
- dpq:
-
Dipyrido[3,2-d:2′,3′-f]quinoxaline
- ESI-MS:
-
Electrospray ionization mass spectrometry
- IPTG:
-
Isopropyl β-d-thiogalactopyranoside
- LB:
-
Luria–Bertani
- 2-ME:
-
2-Mercaptoethanol
- MOPS:
-
3-Morpholinopropanesulfonic acid
- pNPP:
-
p-Nitrophenol phosphate
- phen:
-
1,10-Phenanthroline
- PTP:
-
Protein tyrosine phosphatase
- PTP1B:
-
Protein tyrosine phosphatase 1B
- SAA:
-
Salicylidene anthranilic acid
- SHP-1:
-
Src homology phosphatase 1
- TCPTP:
-
T-cell protein tyrosine phosphatase
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Zhang S, Zhang ZY (2007) Drug Discov Today 12:373–381
Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T (2004) Cell 117:699–711
Zhang ZY (2001) Curr Opin Chem Biol 5:416–423
Andersen JN, Jansen PG, Echwald SM, Mortensen OH, Fukada T, Del Vecchio R, Tonks NK, Moller NPH (2004) FASEB J 18:8–30
Arena S, Benvenuti S, Bardelli A (2005) Cell Mol Life Sci 62:2092–2099
Combs AP, Yue EW, Bower M, Ala PJ, Wayland B, Douty B, Takvorian A, Polam P, Wasserman Z, Zhu WY, Crawley ML, Pruitt J, Sparks R, Glass B, Modi D, McLaughlin E, Bostrom L, Li M, Galya L, Blom K, Hillman M, Gonneville L, Reid BG, Wei M, Becker-Pasha M, Klabe R, Huber R, Li YL, Hollis G, Burn TC, Wynn R, Liu P, Metcalf B (2005) J Med Chem 48:6544–6548
Dewang PM, Hsu NM, Peng SZ, Li WR (2005) Curr Med Chem 12:1–22
Zhang ZY, Lee SY (2003) Expert Opin Investig Drug 12:223–233
Goldstein BJ (2001) Curr Drug Targets Immune Endocr Metabol Disord 1:175–265
Seale AP, de Jesus LA, Kim SY, Choi YH, Lim HB, Hwang CS, Kim YS (2005) Biotechnol Lett 27:221–225
Xie LP, Lee SY, Andersen JN, Waters S, Shen K, Guo XL, Moller NPH, Olefsky JM, Lawrence DS, Zhang ZY (2003) Biochemistry 42:12792–12804
Yuen VG, Caravan P, Gelmini L, Glover N, McNeill JH, Setyawati IA, Zhou Y, Orvig C (1997) J Inorg Biochem 68:109–116
Shrestha S, Bhattarai BR, Lee KH, Cho H (2007) Bioorg Med Chem 15:6535–6548
Sparks RB, Polam P, Zhu W, Crawley ML, Takvorian A, McLaughlin E, Wei M, Ala PJ, Gonneville L, Taylor N, Li Y, Wynn R, Burn TC, Liu PCC, Combs AP (2007) Bioorg Med Chem Lett 17:736–740
Maccari R, Paoli P, Ottana R, Jacomelli M, Ciurleo R, Manao G, Steindl T, Langer T, Vigorita MG, Camici G (2007) Bioorg Med Chem 15:5137–5149
Winter CL, Lange JS, Davis MG, Gerwe GS, Downs TR, Peters KG, Kasibhatla B (2005) Exp Biol Med 230:207–216
Crans DC, Smee JJ, Gaidamauskas E, Yang LQ (2004) Chem Rev 104:849–902
Rehder D (2003) Inorg Chem Commun 6:604–617
Shechter Y, Goldwaser I, Mironchik M, Fridkin M, Gefel D (2003) Coord Chem Rev 237:3–11
Li M, Ding WJ, Baruah B, Crans DC (2008) J Inorg Biochem 102:1846–1853
Thompson KH, Orvig C (2006) J Inorg Biochem 100:1925–1935
Noblia P, Baran EJ, Otero L, Draper P, Cerecetto H, Gonzalez M, Piro OE, Castellano EE, Inohara T, Adachi Y, Sakurai H, Gambino D (2004) Eur J Inorg Chem 322–328
Crans DC, Smee JJ (2004) Comp Coord Chem II 4:175–239
Ghosh T, Bhattacharya S, Das A, Mukherjee G, Drew MGB (2005) Inorg Chim Acta 358:989–996
Thompson KH, Orvig C (2000) Dalton Trans 2885–2892
Mukherjee R, Donnay E.G, Radomski MA, Miller C, Redfern DA, Gericke A, Damron DS, Brasch NE (2008) Chem Commun 3783–3785
Peters KG, Davis MG, Howard BW, Pokross M, Rastogi V, Diven C, Greis KD, Eby-Wilkens E, Maier M, Evdokimov A, Soper S, Genbauffe F (2003) J Inorg Biochem 96:321–330
Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C (1997) J Biol Chem 272:843–851
Nxumalo F, Glover NR, Tracey AS (1998) J Biol Inorg Chem 3:534–542
Lu LP, Zhu ML, Guo ML (unpublished work)
Patel RN, Singh N, Gundla VLN (2006) Polyhedron 25:3312–3318
Dickeson JE, Summers LA (1970) Aus J Chem 23:1023–1027
Amouyal E, Homsi A, Chambron JC, Sauvage JP (1990) J Chem Soc Dalton Trans 1841–1845
Clague MJ, Keder NL, Butler A (1993) Inorg Chem 32:4757–4761
Gans P, Sabatini A, Vacca A (1985) J Chem Soc Dalton Trans 1195–1200
Moghimi A, Alizadeh R, Shokrollahi A, Aghabozorg H, Shamsipur M, Shockravi A (2003) Inorg Chem 42:1616–1624
Martell AE, Chaberek S Jr, Courtney RC, Westerback S, Hyytiainen H (1957) J Am Chem Soc 79:3036–3041
Henry RP, Mitchell PCH, Prue JE (1973) J Chem Soc Dalton Trans 1156–1159
Davis CW (1938) J Chem Soc 2093–2098
Chruscinska EL, Sanna D, Garribba E, Micera G (2008) Dalton Trans 4903–4916
Sheldrick GM (1996) Correction software. University of Göttingen, Göttingen
Sheldrick GM (1997) Program for the solution of crystal structure. University of Göttingen, Göttingen
Sheldrick GM (1997) Program for the refinement of crystal structure. University of Göttingen, Göttingen
Sheldrick GM (1999) SHELXTL/PC, version 5.1. Bruker AXS, Madison
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven JT, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision E.01. Gaussian, Wallingford
Hay PJ, Wadt WR (1985) J Chem Phys 82:270–283
Hay PJ, Wadt WR (1985) J Chem Phys 82:284–298
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Tong Y, Guo ML (2007) J Biol Inorg Chem 12:735–750
Zhu ZC, Sun M, Zhang XY, Liu KX, Shi DL, Li JD, Su JQ, Xu YC, Fu XQ (2007) Chem Res Chin Univ 23:289–296
Li WN, Zhuang Y, Li H, Sun Y, Fu Y, Wu XX, Zhao ZZ, Fu X (2008) Chem Res Chin Univ 24:592–596
Montalibet J, Skorey KI, Kennedy BP (2005) Methods 35:2–8
Ember B, Kamenecka T, LoGrasso P (2008) Biochemistry 47:3076–3084
Mondal S, Rath SP, Rajak KK, Chakravorty A (1998) Inorg Chem 37:1713–1719
Maurya MR, Kumar A, Ebel M, Rehder D (2006) Inorg Chem 45:5924–5937
Root CA, Hoeschele JD, Cornman CR, Kampf JW, Pecoraro VL (1993) Inorg Chem 32:3855–3861
Amin SS, Cryer K, Zhang B, Dutta SK, Eaton SS, Anderson OP, Miller SM, Reul BA, Brichard SM, Crans DC (2000) Inorg Chem 39:406–416
Sasmal PK, Patra AK, Nethaji M, Chakravarty AR (2007) Inorg Chem 46:11112–11121
Zamian JR, Dockal ER, Castellano G, Oliva G (1995) Polyhedron 14:2411–2418
Marques APD, Dockal ER, Skrobot FC, Rosa ILV (2007) Inorg Chem Commun 10:255–261
Westland AD, Tarafder MTH (1981) Inorg Chem 20:3992–3995
Bhattacharya S, Ghosh T (2002) Transit Metal Chem 27:89–94
Soliman AA, Mohamed GG (2004) Thermochim Acta 421:151–159
Raman N, Raja SJ, Joseph J, Raja JD (2007) Russ J Coord Chem 33:7–11
Mathieu M, Van Der Voort P, Weckhuysen BM, Rao RR, Catana G, Schoonheydt RA, Vansant EF (2001) J Phys Chem B 105:3393–3399
Pessoa JC, Cavaco I, Correia I, Costa D, Henriques RT, Gillard RD (2000) Inorg Chim Acta 305:7–13
Ghosh T, Bandyopadhyay C, Bhattacharya S, Mukherjee G (2004) Transit Metal Chem 29:444–450
Dutta SK, Kumar SB, Bhattacharyya S, Tiekink ERT, Chaudhury M (1997) Inorg Chem 36:4954–4960
Stepanenko IN, Krokhin AA, John RO, Roller A, Arion VB, Jakupec MA, Keppler BK (2008) Inorg Chem 47:7338–7347
Wild S, Roglic G, Green A, Sicree R, King H (2004) Diabetes Care 27:1047–1053
Lee S, Wang Q (2006) Med Res Rev 27:553–573
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (grant no. 20471033), the Natural Science Foundation of Shanxi Province (grant no. 20051013), the Overseas Returned Scholar Foundation of Shanxi Province of China in 2006 and 2008, and University of Massachusetts Dartmouth, MA, USA.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
775_2009_496_MOESM1_ESM.pdf
Supplementary material 1 Supporting Information Available: X-ray crystallographic data in CIF format of complex 1 (CCDC708872) and 2 (CCDC707122). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk. Detailed information on the crystal data and structure determination (Table S1), Selected bond lengths and angles for complexes 1 and 2 (Table S2), Unit cell packing diagrams for complexes 1 and 2 (Figs. S1 and S2), UV–vis data for the ligands and the complexes (Table S3), ESI-MS data (Figs. S3, A-E) and molecular modeling of the interactions of complex 2 at the active site of PTP1B (Figs. S4 and S5). (PDF 619 kb)
Rights and permissions
About this article
Cite this article
Yuan, C., Lu, L., Gao, X. et al. Ternary oxovanadium(IV) complexes of ONO-donor Schiff base and polypyridyl derivatives as protein tyrosine phosphatase inhibitors: synthesis, characterization, and biological activities. J Biol Inorg Chem 14, 841–851 (2009). https://doi.org/10.1007/s00775-009-0496-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-009-0496-6