Abstract
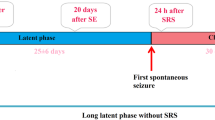
X-ray fluorescence microscopy was applied for topographic and quantitative elemental analysis within the areas of the rat brain that undergo neurodegenerative changes in consequence of pilocarpine-induced seizures. Significant changes in levels of selected elements were observed in epileptic animals. They included an increased tissue content of Ca in the CA1 and CA3 regions of the hippocampus and in the cerebral cortex. The opposite relation was observed for the Cu level in the dentate gyrus and for Zn in the CA3 region of the hippocampus and in the dentate gyrus.




Similar content being viewed by others
References
Hamed SA, Abdellah MM (2004) Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients. J Pharmacol Sci 96:349–359
Lynes MA, Kang YJ, Sensi SL et al (2007) Heavy metal ions in normal physiology, toxic stress, and cytoprotection. Ann N Y Acad Sci 1113:159–172
Fraga CG (2005) Relevance, essentially and toxicity of trace elements in human health. Mol Aspects Med 26:235–244
Formigari A, Irato P, Santon A (2007) Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol 146:443–459
Bush AI (2000) Metals and neuroscience. Curr Opin Chem Biol 4:184–191
Sayre LM, Perry G, Smith MA (1999) Redox metals and neurodegenerative disease. Curr Opin Chem Biol 3:220–225
Campbell A, Smith MA, Sayre LM et al (2001) Mechanisms by which metals promote events connected to neurodegenerative diseases. Brain Res Bull 55:125–132
Hamed SA, Abdellah MM (2004) Trace elements and electrolytes homeostasis and their relation to antioxidant enzyme activity in brain hyperexcitability of epileptic patients. J Pharm Sci 96:349–359
Papavasiliou PS, Miller ST (1983) Generalized seizures alter the cerebral and peripheral metabolism of essential metals in mice. Exp Neurol 82:223–236
Carl GF, Critchfield JW, Thompson JL et al (1989) Effect of kainate-induced seizures on tissue trace element concentrations in the rat. Neuroscience 33:223–227
Carl GF, Critchfield JW, Thompson JL et al (1990) Genetically epilepsy-prone rats are characterized by altered tissue trace element concentrations. Epilepsia 31:247–252
Hirate M, Takeda A, Tamano H et al (2002) Distribution of trace elements in the brain of EL (epilepsy) mice. Epilepsy Res 51:109–116
Setkowicz Z, Janeczko K (2003) Long-term changes in susceptibility to pilocarpine-induced status epilepticus following neocortical injuries in the rat at different developmental stages. Epilepsy Res 53:216–224
Setkowicz Z, Nowak B, Janeczko K (2006) Neocortical injuries at different developmental stages determine different susceptibility to seizures induced in adulthood. Epilepsy Res 68:255–263
Turski L, Cavalheiro EA, Czuczwar SJ et al (1987) The seizures induced by pilocarpine: behavioral, electroencephalographic and neuropathological studies in rodents. Pol J Pharmacol Pharm 39:545–555
Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW (2007) Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol 35:984–999
Janssens KH, Rindby A, Adams F (2000) Microscopic X-ray fluorescence analysis. Wiley, Chichester
Szczerbowska-Boruchowska M, Lankosz M, Ostachowicz J et al (2004) Topographic and quantitative microanalysis of human central nervous system tissue using synchrotron radiation. X-ray Spectrom 33:3–11
Tomik B, Chwiej J, Szczerbowska-Boruchowska M et al (2006) Implementation of X-ray fluorescence microscopy for investigation of elemental abnormalities in amyotrophic lateral sclerosis. Neurochem Res 4:321–331
Snigireva I, Snigirev A (2006) X-ray microanalytical techniques based on synchrotron radiation. J Environ Monit 8:33–42
Setkowicz Z, Mazur A (2006) Physical training decreases susceptibility to subsequent pilocarpine-induced seizures in the rat. Epilepsy Res 71:142–148
Setkowicz Z, Ciarach M (2007) Neuroprotectants FK-506 and cyclosporin A ameliorate the course of pilocarpine-induced seizures. Epilepsy Res 73:151–155
Paxinos G, Watson C (1989) The rat brain in stereotaxic coordinates. Academic Press, Australia
Falkenberg G, Clauss O, Tschentscher T (2001) X-ray optics for the microfocus beamline L. In: HASYLAB annual report 2001. Available at http://hasyweb.desy.de/science/annual_reports/2001_report/part1/intern/5720.pdf
Vekemans B, Vincze L, Somogyi A, Drakopoulos M, Kempenaers L, Simionovici A, Adams F (2003) Quantitative X-ray fluorescence analysis at the ESRF ID18F microprobe. Nucl Instrum Methods B 199:396–401
Currie LA (1968) Limits of qualitative detection and quantitative determination. Anal Chem 40:586–593
Takeda A, Hanajima T, Ijiro H et al (1999) Release of zinc from the brain of El (epilepsy) mice during seizure induction. Brain Res 828:174–178
Takeda A, Hirate M, Tamano H et al (2003) Susceptibility to kainate-induced seizures under dietary zinc deficiency. J Neurochem 85:1575–1580
Takeda A, Tamano H, Nagayoshi A et al (2005) Increase in hippocampal cell death after treatment with kainate in zinc deficiency. Neurochem Int 47:539–544
Ren MQ, Ong WY, Makjanic J et al (1999) Changes in calcium and iron levels in the brains of rats during kainate induced epilepsy. Nucl Instrum Methods B 158:418–423
Lankosz M, Holynska B, Pella PA (1993) Experimental verification of a Monte Carlo method for X-ray microfluorescence analysis of small particles. X-ray Spectrom 22:54–57
Pillay AE (2001) Analysis of archaeological artefacts: PIXE, XRF or ICP-MS? J Radioanal Nucl Chem 247:593–595
Ali M (2004) PIXE and RIXRF comparison for applications to biological sample analysis. Nucl Instrum Methods B 222:567–576
Glass M, Dragunow M (1995) Neurochemical and morphological changes associated with human epilepsy. Brain Res Rev 21:29–41
Siesjö BK, Bengtsson F (1989) Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab 9:127–140
Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34:325–337
Chwiej J, Szczerbowska-Boruchowska M, Lankosz M et al (2005) Preparation of tissue samples for X-ray fluorescence microscopy. Spectrochim Acta B 60:1531–1537
Parkin G (2004) Chemistry. Zinc–zinc bonds: a new frontier. Science 305:1117–1118
Williamson A, Spencer D (1995) Zinc reduces dentate granule cell hyperexcitability in epileptic humans. Neuroreport 6:1562–1564
Pei Y, Zhao D, Huang J, Cao L (1983) Zinc-induced seizures: a new experimental model of epilepsy. Epilepsy 24:169–176
Bossy-Wetzel E, Schwarzenbacher R, Lipton SA (2004) Molecular pathways to neurodegeneration. Nat Med 10:2–9
Vogt K, Mellor J, Tong G et al (2000) The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26:187–196
Leite JP, Garcia-Cairasco N, Cavalheiro EA (2002) New insights from the use of pilocarpine and kainate models. Epilepsy Res 50:93–103
Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6:171–180
Sahin D, Ilbay G, Ates N (2003) Changes in the blood–brain barrier permeability and in the brain tissue trace element concentrations after single and repeated pentylenetetrazole-induced seizures in rats. Pharmacol Res 48:69–73
Acknowledgments
This work was supported by the Polish Ministry of Science and Higher Education and the following grants: IA-SFS/94/2007, N303 052 31/1626, RII3-CT-2004-506008 (IA-SFS), and DESY-D-I-20070053 EC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chwiej, J., Winiarski, W., Ciarach, M. et al. The role of trace elements in the pathogenesis and progress of pilocarpine-induced epileptic seizures. J Biol Inorg Chem 13, 1267–1274 (2008). https://doi.org/10.1007/s00775-008-0411-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-008-0411-6