Abstract
Removal of metal compounds from wastewater using processes where metals can be removed and valuable chemicals recycled is of significant industrial importance. Chelating surfactants are an interesting group of chemicals to be used in such applications. Carboxylated polyamines are a promising group to be used in such processes. To apply carboxylated polyamines as chelating surfactants, detailed knowledge of the solution chemistry, including complex formation, kinetics and structures of pure fundamental systems, is required. In this study zinc(II) alkyl-N-iminodiacetate systems with varying length of the alkyl chain have been studied. Acidic and stability constants have been studied by potentiometry, and the structures of both solids and aqueous solutions have been determined by EXAFS. Zinc(II) forms two strong complexes with alkyl-N-iminodiacetates in aqueous solution. In an attempt to determine the acidic constants of these complexes, the deprotonation of the nitrogen atom in the complex bound ligands, it was observed that this reaction is very slow and no accurate values could be obtained. The bis(alkyl-N-iminodiacetato)zincate(II) complexes take, however, up two protons in the pH region 3–7, which means that this complex is approximately singly protonated in the pH region 3–7 and doubly protonated at pH < 3. The bis(n-hexyl-N-iminodiacetato)zincate(II) complex at pH = 13 has a distorted octahedral configuration with four short strong Zn–O bonds at 2.08(1) Å, while the Zn–N bonds are weaker at much longer distance, 2.28(2) Å. Similar configurations are also found in most reported structures of zinc(II) complexes with carboxylated amines/polyamines. The singly protonated complex seems to be five-coordinate, with four Zn–O bond distances at ca. 2.03 Å, and a single Zn–N bond distance in the range 2.15–2.25 Å. The relationship between the structure of the protonated bis(n-hexyl-N-iminodiacetato)zincate(II) complex and the slow kinetics in the region pH = 3–7 are discussed.
Similar content being viewed by others
1 Introduction
For environmental reasons, the cleaning of wastewater from industries and purifying plants from heavy metals is of utmost importance. It is essential to develop cost-effective processes where metals can be separated and recycled or taken care of, and the collector chemical recycled. This has caused an increasing interest in efficient metal binding ligands with surface active properties, so called chelating surfactants. Chelating surfactants must fulfil several requirements to be applied in industrial processes and able to reach the commercial market: (i) being biodegradable and non-toxic for the environment, (ii) the complexation of metal ions must be strong, and at the same time reversible in such a way that both surfactant and metal ion can be separated and recycled, (iii) the synthesis must be readily feasible with environmentally acceptable chemicals, and (iv) it must be possible to separate the metal ions by changing some physico-chemical parameters such as pH, temperature or ionic strength. Carboxylated alkyl polyamines are a group of chemicals used in many technical applications and they fulfil the requirements for chelating surfactants given above. However, these commercial products contain several compounds with varying alkyl chain length, and different numbers of amine and carboxylate groups. This makes it more or less impossible to perform accurate physical–chemical studies on such products. However, their chemical behavior can be estimated with reasonable accuracy from the properties of well-defined pure model compounds, such as alkyl-N-iminodiacetates.
The zinc(II) ion, with d10 electron configuration, is classified as a hard to borderline electron-pair acceptor [1,2,3,4], with no preferred configuration of the complexes [5]. The structure of zinc(II) complexes with oxygen and/or nitrogen ligand atoms in the solid state have been characterized in a large number of compounds with coordination numbers from four to seven [5]. Zinc(II) has been reported to form two fairly strong complexes in aqueous solution with methyl-N-iminodiacetate [6,7,8], with, for example, log10 β1 = 7.44 and log10 β2 = 13.70 (0.5 mol·dm−3 NaClO4 as supporting electrolyte) [6]. The formation of both complexes takes place with a strong entropy contribution, as expected in the formation of chelate complexes [9, 10]. Slightly more stable complexes are reported for n-propyl- and n-butyl-N-iminodiacetate [11], with log10 β1 = 8.00 and log10 β2 = 14.55, and log10 β1 = 8.12 and log10 β2 = 14.88, respectively (0.1 mol·dm−3 KNO3). A survey of reported stability constants of zinc(II)–iminodiacetate and alkyl-N-iminodiacetate complexes is given in Table S1. In the crystal structure of sodium bis(iminodiacetato)zinc(II) heptahydrate, Na2[Zn(NH(CH2COO)2)2]·7H2O, zinc binds two oxygens and one nitrogen from each iminodiacetate ligand at 2.109 and 2.120 Å, respectively, in octahedral fashion [12].
The aim of this study is to get a more detailed understanding of the physical–chemical and structural requirements for complex formation in the zinc–alkyl-N-iminodiacetate system by varying the length of the alkyl chain to determine the composition and stability of the complexes formed and their configurations. The results showed that the role of the imine nitrogen in the complex formation and the protolytic properties of alkyl-N-iminodiacetatozincate complexes in aqueous solution is of particular interest. In order to get a correct description of the complexes it is essential to know whether the nitrogen in the alkyl-N-iminodiacetate ligand binds to the metal or not. The structures of the alkyl-N-iminodiacetatozincate complexes in aqueous solution and in the solid state have been investigated by EXAFS spectroscopy. An attempt to determine the stability and acidic constants of zinc(II)–alkyl-N-iminodiacetate systems in aqueous solution was made with potentiometry using pH and zinc amalgam electrodes simultaneously.
2 Experimental
2.1 Chemicals
Methyl-N-iminodiacetic acid, CH3N(CH2COOH)2, (Aldrich, 99%), perchloric acid 70–72% (Merck, p.a.), sodium hydroxide solution (Merck, Titrisol), sodium perchlorate monohydrate, NaClO4·H2O (Merck, p.a., ≥ 99.0%) and zinc(II) perchlorate hexahydrate, [Zn(H2O)6](ClO4)2, (GFS Chemicals, ≥ 99.8%) were used as purchased. n-Hexyl- and n-octadecyl-N-iminodiacetic acid, RN(CH2COOH)2, R = n-C6H13 and n-C18H37, were synthesized and purified by Hans Oskarsson, Nouryon Surface Chemistry AB, as described elsewhere [13], and were used without further treatment. Zinc amalgam was prepared by dissolving 6.11 g of zinc metal (Alfa, ≥ 99.99%) in 227.7 g liquid mercury (Kebo, ≥ 99.99%) in a 500 mL round flask under argon atmosphere and was left to mix at ca. 333 K for 48 h [14]. The amalgam was slowly cooled down to room temperature over 24 h and a two-phase amalgam was formed, where the liquid phase was used in the potentiometric studies. The water used was deionized and filtered by a Milli-Q filter system giving water with 18.2 MΩ·cm resistance.
2.2 Sample Preparation
For acid–base titrations, the titrands were mixed from stock-solutions of 0.100 mol·dm−3 zinc(II) perchlorate and 0.100 mol·dm−3 methyl-N-iminodiacetic acid or 0.020 mol·dm−3 n-hexyl-N-iminodiacetic acid at the isoelectric pH. The zinc(II) concentrations were held between 5 × 10−3 and 5 × 10−2 mol·dm−3 and the ionic strength was maintained at 0.1 mol·dm−3 with sodium perchlorate as supporting electrolyte. 1.000 or 0.100 mol·dm−3 hydrochloric acid or sodium hydroxide solutions were used as titrants.
The EXAFS samples were prepared from mixing stoichiometric amounts of stock solutions of zinc(II) perchlorate and alkyl-N-iminodiacetic acid pH adjusted by one mole equivalent of sodium hydroxide, if not studied under isoelectric pH conditions. The samples were ultrasonicated for several hours. In all samples a white precipitate was formed. The aqueous samples were studied after centrifugation to remove solid particles. The solutions were contained in cells made of 2 mm Teflon spacers and 6 μm X-ray polypropylene film windows held together with titanium frames. For the solid samples the precipitate was separated, dried and mortared/mixed with ca. 60 weigh% boron nitride. The cells used for the solid samples were typically 1.0 mm Ag-frames covered by Mylar tape.
2.3 Potentiometry
The experimental setup was two jacketed glass cells (ca 25 ml, Metrohm) connected to a third common jacketed reference cell by salt-bridges containing 0.1 mol·dm−3 sodium perchlorate. All cells contained the same zinc solution from the start. The free zinc(II) concentration was determined by Zn–amalgam electrodes contained in glass spoons, where platinum wire electrodes were immersed into the Zn-amalgam. The potentials were measured with a high-resistance voltmeter, Keithley 6517B, with an accuracy better than 0.1 mV. pH was recorded by an Orion Research EA 940 potentiometer with a Mettler Toledo InLab®422 electrode or an Orion 9102AP pH electrode. The pH electrodes were calibrated with Orion standards at pH = 4.01, 7.00 and 10.01. The zinc amalgam electrodes were calibrated with zinc solutions made from dissolution of heated zinc oxide, ZnO, in perchloric acid, in the concentration range 10−5–10−2 mol·dm−3. The zinc amalgam electrodes were found to follow Nernstian slope with a value of 29.6 mV. All measurements were performed at ambient room temperature, 295 ± 1 K. The titrant solution was manually added from an analogue burette. The titrations of the zinc(II)–n-alkyl-N-iminodiacetate systems showed that the time to reach stable pH and voltmeter readings were ca. 1–5 min. except in the pH range 3–8 where the time required to reach stable pH values and zinc electrode potentials was at least 15 min.
The evaluation of stability constants was made numerically with the use of the program EMFALL [15], and acidic constants reported previously [13]. However, the data evaluation showed that the potentials drifted slowly with time, and no accurate determinations of the stability constants from either the pH or zinc electrode measurements were possible for any of the studied zinc–alkyl-N-iminodiacetate systems.
2.4 EXAFS
The Zn K-edge EXAFS measurements were performed at the Stanford Synchrotron Radiation Lightsource (SSRL), USA. SSRL did operate at 3.0 GeV and a maximum current of 100 mA. The EXAFS station at the wiggler beam line 4–1, old station, was equipped with a Si[220] double crystal monochromator. A Lytle detector with krypton gas was used for fluorescence emission measurements. X-ray absorption spectroscopy data were simultaneously measured in both fluorescence and transmission modes. Internal calibration was made simultaneously with a zinc metal foil. The energy of the first inflection point on the zinc K absorption edge is defined as 9659 eV [16]. Higher order harmonics were discarded by detuning the second monochromator crystal to 50% of maximum intensity at the end of the scans. The data treatment and model fitting of the EXAFS data was carried out using the EXAFSPAK program package [17], using standard procedures for pre-edge subtraction and spline removal [18]. The resulting EXAFS functions have been curve-fitted by calculated model functions using ab initio calculated EXAFS phase and amplitude parameters from FEFF7 (ver. 7.02) [19, 20]. Theoretical back-scattering amplitude functions were calculated for each back-scattering shell of oxygen, nitrogen, carbon and metal atoms, including multiple scattering paths. Each set of EXAFS data was curve-fitted using paths from each back-scattering shell and the Fourier transformed EXAFS function shows the density distribution function of the scattering path distances.
3 Results
3.1 Protolytic Properties of Complexes
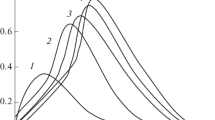
Solutions of zinc(II) perchlorate and methyl-N-iminodiacetic acid (MIDA) in molar ratios 1:1, 1:2 and 1:3 were titrated with a sodium hydroxide solution. The titrand solutions were obtained by mixing zinc(II) perchlorate and MIDA solutions at the isoelectric pH, where an average of one acetic acid group per ligand is deprotonated, while the imine nitrogen is fully protonated giving a neutral zwitterion. From the titration functions the complex stoichiometry of two alkyl-N-iminodiacetic acid ligands per zinc(II) ion was obtained. When the excess of ligand is two-fold or larger the solutions were perfectly clear. The titration functions are shown in Fig. 1.
pH titration functions from the acid and base titration of aqueous solutions of zinc(II) methyl-N-iminodiacetate with the molar ratios 1:1, 1:2 and 1:3, and of zinc(II) hexyl-N-iminodiacetate with the molar ratio 1:5. The measurements were performed at 295 ± 1 K, at ionic strength of 0.10 mol·dm−3 with sodium perchlorate as supporting electrolyte
At titrations of an aqueous solution with a zinc(II)/MIDA ratio of 1:1, the released proton from the coordinated acidic acetic acid groups reacts with one mole equivalent sodium hydroxide. Immediately after the equivalence point at ca. pH = 6, flocculation of zinc hydroxide was observed, showing a hydrolysis plateau corresponding to about one half mole equivalents of zinc(II). Above the last equivalence point at pH around 9 there are no further protolytic species in the sample. At the stoichiometry ratio zinc(II)/MIDA equal to 1:2, all zinc(II) is present as the second complex, ZnL2. This is observed as two mole equivalents of released protons from coordinated acetic acid groups in the pH range 3–5. This is followed by the deprotonation of two mole equivalents of the amine nitrogen atoms in the pH range 3–7 (Fig. 1). At pH values above the equivalence point, ca. 8, there are no further acidic protons in the system. At pH values above 12 some hydrolysis product starts to precipitate. At the stoichiometry ratio zinc(II)/MIDA equal to 1:3 all zinc(II) is present as the second complex and one mole equivalent of methyl-N-iminodiacetic acid remains uncomplexed. In the titration function three mole equivalents of alkyl-N-iminoacetic acid are reacted followed by the deprotonation of two mole equivalents of complexed imine nitrogen atoms in the pH range 3–7. Above the equivalence point at pH ≈ 9.5 no further protolytic reactions were observed, but also in this case a hydrolysis product precipitates at pH > 12.
However, most attention should be paid to the titration functions in the pH region between 3 and 7 where the imine nitrogen atoms in the bound ligands are deprotonated. The buffering region is undefined and smeared out, most probably because of slow kinetics resulting in a poorly defined composition of the solution. Despite waiting for up to 30 min. for each pH reading and the pH values seemed stable, the pH titration curves show clearly that thermodynamic equilibrium was not reached (Fig. 1). Below pH = 3 and above pH = 9, equilibria were reached rapidly, except at the 1:1 stoichiometry, where solid zinc hydroxide was formed. To check the validity of the discussion above, a 1:5 stoichiometry sample of zinc(II) and hexyl-N-iminodiacetic acid was titrated, and the interpretation was similar. The bis(methyl-N-iminodiacetato)zincate(II) complex has two acidic constants in the region 10−7–10−3 but they have not been possible to determine them accurately due to the unstable pH and zinc potential readings in this pH region.
3.2 EXAFS
A number of zinc(II) alkyl-N-iminodiacetate complexes in both aqueous solution and the solid state have been studied by means of EXAFS. The samples differ in stoichiometric composition, pH and the alkyl chain length of the ligand. The EXAFS functions of all samples show two major back-scattering contributions to the EXAFS function, observed as double peaks at k = 5–6 and 9–10 Å−1 in the EXAFS functions, Fig. 2. The Fourier transforms of the EXAFS functions show a large and narrow peak at 2.0 Å (after phase correction), corresponding to the first back-scattering shell of oxygen atoms and, in some cases, the nitrogen atoms as well, and small peaks at ca. 2.8 Å corresponding to a back-scattering shell of carbon atoms, and at 3.0 Å corresponding to the 3-leg Zn–O–C multiple scattering, Fig. S1.
Fit of EXAFS data of (a) aqueous solution of zinc(II) di-n-hexyl-N-iminodiacetate complexes at pH = 1.5, (b) solid zinc(II) di-n-hexyl-N-iminodiacetate complex precipitated at pH = 1.5, (c) (a) aqueous solution of zinc(II) dimethyl-N-iminodiacetate complex at pH = 5.5, (d) solid zinc(II) dimethyl-N-iminodiacetate complex precipitated at pH = 5.5, (e) aqueous solution of zinc(II) di-nhexyl-N-iminodiacetate complex at pH = 5.5, (f) solid zinc(II) di-n-hexyl-N-iminodiacetate complex precipitated from aqueous solution at pH = 5.5, (g) solid zinc(II) di-dodecyl-N-iminodiacetate complex precipitated at pH = 7.5, (h) solid zinc(II) di-octadecyl-N-iminodiacetate complex precipitated at pH = 6.5, and (i) solid zinc(II) di-n-hexyl-N-iminodiacetate complex precipitated at pH = 13.0. The solid lines represent the experimental data and dashed lines the model using the parameters given in Table 1.
The bis(n-hexyl-N-iminodiacetato)zincate(II) complex in aqueous solution at pH ≈ 13 is six-coordinate in a distorted octahedral fashion with four short Zn–O bonds at 2.08(1) Å and two Zn–N bonds much more weakly bound at 2.29(2) Å. The Zn⋯C distance was determined to 2.91(2) Å. When the pH is decreased to 5.5 and it can be assumed that, on average, the nitrogen in one of the ligands is protonated and the mean coordination number is expected to decrease to five. This solution is fairly dilute, which causes that the data to be significantly more noisy than the other EXAFS spectra in this study. The mean value of approximately four Zn–O bond distances was determined 2.04(3) Å, and the Zn–N distance was refined to 2.13(10) Å. An aqueous solution of bis(n-hexyl-N-iminodiacetato)zincate(II) ion at pH ≈ 5.5 has a very similar structure to the methyl analogue. When pH is further decreased to 1.5, the ligand is fully protonated, and the dominating zinc species is the hexahydrated zinc(II) ion. However, it is not possible, from the present data, to estimate if any n-hexyl-N-iminodiacetate ligand is monodentately weakly bound to zinc(II) or not.
The solid complexes precipitated from solutions at neutral or slightly acidic aqueous solutions are on average four- or five-coordinated with mean Zn–O bond distances of 2.01–2.05 Å. This strongly indicates that one ligand is strongly bound with two short Zn–O bonds and a longer Zn–N one, while it is not possible, from the present data, to judge whether a second alkyl-N-iminodiacetate ligand with a protonated nitrogen is bound as a 8-membered chelate ring or if it is bridging between two zinc(II) sites. The solid phase of zinc(II) n-octadecyl-N-iminodiacetate shows, besides the normal five-coordination, a Zn⋯Zn distance at 3.90(2) Å, showing the rigid structural arrangement of the iminodiacetate groups in the surface of n-dodecyl-N-iminodiacetate aggregates. The fit of the EXAFS data are shown in Fig. 2, and the refined structure parameters are summarized in Table 1 and the complete set structure parameters in Table S1.
4 Discussion
Zinc(II) forms two stable complexes with the alkyl-N-iminodiacetate in aqueous solution [6,7,8,9,10,11], which is confirmed in this study. In the alkyl-N-iminodiacetatozincate(II) complexes the nitrogen atoms are protonated in acidic solution, pH < 3, while they are deprotonated above pH ≈ 7. In the pH range 3–7 protonation/deprotonation of the imino nitrogen takes place in complexed alkyl-N-iminodiacetate ligands. The experimentally obtained pH values and zinc potentials are very unstable and it take a very long time to reach equilibrium. This shows that the protonation/deprotonation step is very slow, and it seems very unlikely that any well-defined distribution of the [Zn(R-NH(CH3COO)2] [Zn(R-N(CH3COO)2)(R-NH(CH3COO)2)]− and [Zn(R-NH(CH3COO)2]2− complexes is present. It seems very likely that the protonation and deprotonation steps are accompanied by significant structural rearrangements of the complexes. When the nitrogen atom in a complex bound alkyl-N-iminodiacetate ligand is deprotonated it is converted from one 8-membered chelate ring to two 5-membered ones. At this step the nitrogen atom in the ligand has to find and bind to zinc. The structure data show that the nitrogen is weakly bound to zinc. The energy gained by the Zn–N binding is likely to be of the same order as the energy required to break the N–H bond and rearrange from an 8- to a 5-membered ring. Therefore, the driving force for deprotonation is weak and it takes long time for the involved species to reach equilibrium. The results in this study strongly indicate that the configuration change between two 5-membered and one 8-membered chelate ring(s) is a slow step, probably due to that the nitrogen, including the entire ligand, and has difficulties to transfer between binding two or three donor atoms in the ligand, most probably including some rotation barriers.
The bis(n-hexyl-N-iminodiacetato)zincate(II) complex at pH = 13 has a distorted octahedral configuration with four short relatively strong Zn–O bonds at 2.08(1) Å, while the Zn–N bonds are weaker at much longer distance, ca. 2.28(2) Å, Tables 1 and S1. In the structure of the bis(iminodiacetato)zincate(II) complex in the solid state [12] the nitrogen atoms are bound equally strongly as the oxygens, which may be due to weaker hydration and/or lattice effects. The only similar structure in the solid state is the zinc(II)–EDTA complex where the mean Zn–O and Zn–N bond distances are 2.077 and 2.174 Å, respectively [21]. This is typical for zinc(II) complexes with carboxylated amines/polyamines where the Zn–O bond distances are significantly shorter and stronger than the Zn–N, Table S3. In neutral and weakly acidic solutions the coordination number of the protonated bis(n-hexyl-N-iminodiacetato)zincate(II) complex decreases to five in aqueous solution, and to four in some solid samples, Tables 1 and S1. The reason for the lower coordination number in the solid state is probably lattice effects, as complexes with high symmetry as tetrahedrons and octahedrons are favored. The crystal structure of bis(iminodiacetato)zincate(II) tetrahydrate, obviously precipitated at low pH, shows that the imino nitrogens are protonated and zinc forms two 8-membered chelate complexes with diacetateammonium ions [22]. Zinc(II) is five-coordinated in the crystal structure of diaqua-(2-ethylphenylamino-N,N-diacetato)zinc(II) complex with mean Zn–O bond distances of 2.002 Å, while the Zn–N bond is as long as 2.258 Å [23]. In spite of a limited amount of structural data for zinc(II) complexes of ligands containing both carboxylate and amine groups, it is obvious that the zinc(II) ion, regarded as borderline electron-pair acceptor, binds the oxygen donor atoms much more strongly than the amine/imine nitrogen ones.
n-Octadecyl-N-iminodiacetate forms aggregates in water due the formation of short strong hydrogen bonds (SSHBs) further stabilized by van der Waals forces between the n-octadecyl chains [13]. The present EXAFS data show that zinc binds to the iminodiacetate groups in such aggregates in a similar way as to monomeric alkyl-N-iminodiacetate groups in aqueous solution, even though steric restriction may be present.
5 Conclusions
Zinc(II) forms two stable complexes with alkyl-N-iminodiacetate in aqueous solution. At pH < 3 both complex bound alkyl-N-iminodiacetate ligands are protonated on the nitrogen atom causing the ligands to form 8-membered chelate rings. With the deprotonation of the nitrogen atom, the nitrogen atom binds weakly to zinc and two 5-membered chelate complexes are formed. At pH > 8 the ligands are deprotonated, and the Zn–O and Zn–N bond distances in the [Zn(RNCH2COO)2]2− complex in aqueous solution are 2.08 and 2.29 Å, respectively, in a distorted octahedral configuration. In the pH range 3–7, on average, one of the ligands is protonated resulting 5-coordinated complexes. However, in this pH region the potentials in potentiometric measurements are unstable indicating poorly defined complex composition and difficulties for the 8-membered chelate ring to rearrange into two 5-membered ones.
References
Ahrland, S., Chatt, J., Davies, N.R.: The relative affinities of ligand atoms for acceptor molecules and ions. Q. Rev. Chem. Soc. 12, 265–276 (1958)
Pearson, R.G.: Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963)
Pearson, R.G.: Hard and soft acids and bases, HSAB, Part I: Fundamental principles. J. Chem. Ed. 45, 581–587 (1968a)
Pearson, R.G.: Hard and soft acids and bases, HSAB, Part II: underlaying theories. J. Chem. Ed. 45, 643–648 (1968b)
Bock, C.W., Katz, A.K., Glusker, J.P.: Hydration of zinc ions: a comparison with magnesium and beryllium ions. J. Am. Chem. Soc. 117, 3754–3765 (1995)
Gaizer, F., Lazar, J., Kiss, J., Poczik, E.: Protonation and complex formation equilibria of N-(phenylcarbamoylmethyl)iminodiacetic acid derivatives—I. The complexes of HIDA and diethylcarbamoyl-MIDA. Polyhedron 11, 257–264 (1992)
Mirti, P., Gennaro, M.: Nuclear magnetic resonance investigation of ligand exchange reactions in the zinc(II) ion—N-methyliminodiacetic acid system. J. Nucl. Inorg. Chem. 39, 1259–1264 (1977)
Schwarzenbach, G., Kampitsch, E., Steiner, R.: Komplexone II Das komplexbildungsvermögen von iminodiessigsäure, methyliminodiessigsäure, aminomalonsäure und aminomalonsäurediessigsäure. Helv. Chim. Acta 28, 1133–1143 (1945)
Anderegg, G.: Komplexone XXXVIII. Reaktionsenthalpie und -entropie bei der bildung der 1:1-und 1:2-metallkomplexe von methyliminodiessigsäure. Helv. Chim. Acta 48, 1718–1721 (1965a)
Anderegg, G.: Komplexone XXXIX. Die bildungs-enthalpie und -entropie der metallkomplexe des diäthylentriaminpentaacetat-ions. Helv. Chim. Acta 48, 1722–1725 (1965b)
Israeli, M., Petit, L.: Co-ordination of silver(I) to olefinic bonds complex formation between cobalt(II), nickel(II), copper(II), zinc(II), cadmium(II), and silver(I) and some unsaturated derivatives of acetic and iminodiacetic acids. J. Chem. Soc. Dalton Trans. 5, 414–417 (1975)
Hu, S.-Z.: On the incorrect assignment of space groups III. Revised P1 structures. Chin. J. Struct. Chem. 19, 153–157 (2000)
Häggman, L., Lindblad, C., Oskarsson, H., Ullström, A.-S., Persson, I.: The influence of short strong hydrogen bonding on the structure and the physicochemical properties of alkyl-N-iminodiacetic acids in solid state and aqueous systems. J. Am. Chem. Soc. 125, 3631–3641 (2003)
Persson, H.: The complex formation in the zinc thiosulfate and the cadmium thiosulfate systems. Acta Chem. Scand. 24, 3739–3750 (1970)
Sandell, A.: EMFALL—A Program for Evaluation of Stability Constants from Potentiometric Data. Physical Chemistry 1. Lund University, Lund, personal communication; the source code is available from corresponding author of this paper.
Thompson, A., Attwood, D., Gullikson, E., Howells, M., Kim, K.-J., Kirz, J., Lindau, I., Liu, Y., Pianetta, P., Robinson, A., Scofield, J., Underwood, J., Vaughan, D., Williams, G., Winick, H.: X-ray Data Booklet. Lawrence Berkeley National Laboratory, Berkeley, 3rd rev. LBNL/PUB-490 Rev. 3 (2009). https://xdb.lbl.gov/xdb-new.pdf. Accessed 16 Oct 2020.
George, G.N., Pickering, I.J.: EXAFSPAK—A Suite of Computer Programs for EXAFS Analysis, SSRL. Stanford University, Standford (1993)
Calvin, S.: XAFS for Everyone. CRC Press, Boca Raton (2013)
Zabinsky, S.I., Rehr, J.J., Ankudinov, A.L., Albers, R.C., Eller, M.J.: Multiple-scattering calculations of X-ray absorption spectra. Phys. Rev. B 52, 2995–3009 (1995)
Ankudinov, A.L., Rehr, J.J.: Relativistic calculations of spin-dependent X-ray absorption spectra. Phys. Rev. B 56, R1712–R1716 (1997)
Sysoeva, T.F., Starikova, Z.A., Leonteva, M.V., Dyatlova, N.M.: Crystal structure of bis(ethylenediamine)cuprate ethylenediaminetetraacetatezinc(ii) tetrahydrate. Zh. Strukt. Khim. 31, 140–143 (1990)
Sinkha, U.C., Kramarenko, F.G., Polynova, T.N., Porai-Koshits, M.A., Mitrofanova, N.D.: Crystal structure of acidic Zn(II)–diiminodiacetate tetrahydrate. J. Struct. Chem. 16, 131–131 (1975)
Hidalgo, M.A., Suarez-Varela, J., Avila-Roson, J.C., Martin-Ramos, J.D., Romerosa, A.: Diaqua(2-ethylphenyliminodiacetato-N,O,O’)zinc(II). Acta Crystallogr. C 52, 810–812 (1996)
Acknowledgements
The financial support from Nouryon Surface Chemistry AB, Stenungsund, is gratefully acknowledged. The use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Funding
Open access funding provided by Swedish University of Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Häggman, L., Lindblad, C., Cassel, A. et al. Complex Formation Between Zinc(II) and Alkyl-N-iminodiacetic Acids in Aqueous Solution and Solid State. J Solution Chem 49, 1279–1289 (2020). https://doi.org/10.1007/s10953-020-01031-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01031-w