Abstract
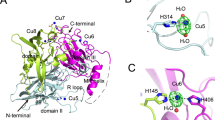
Site-directed mutagenesis has been used to replace Met502 in CotA laccase by the residues leucine and phenylalanine. X-ray structural comparison of M502L and M502F mutants with the wild-type CotA shows that the geometry of the T1 copper site is maintained as well as the overall fold of the proteins. The replacement of the weak so-called axial ligand of the T1 site leads to an increase in the redox potential by approximately 100 mV relative to that of the wild-type enzyme (E 0=455 mV). However the M502L mutant exhibits a twofold to fourfold decrease in the k cat values for the all substrates tested and the catalytic activity in M502F is even more severely compromised; 10% activity and 0.15–0.05% for the non-phenolic substrates and for the phenolic substrates tested when compared with the wild-type enzyme. T1 copper depletion is a key event in the inactivation and thus it is a determinant of the thermodynamic stability of wild-type and mutant proteins. Whilst the unfolding of the tertiary structure in the wild-type enzyme is a two-state process displaying a midpoint at a guanidinium hydrochloride concentration of 4.6 M and a free-energy exchange in water of 10 kcal/mol, the unfolding for both mutant enzymes is clearly not a two-state process. At 1.9 M guanidinium hydrochloride, half of the molecules are in an intermediate conformation, only slightly less stable than the native state (approximately 1.4 kcal/mol). The T1 copper centre clearly plays a key role, from the structural, catalytic and stability viewpoints, in the regulation of CotA laccase activity.






Similar content being viewed by others
References
Messerschmidt A (1997) Multi-copper oxidases. World Science, Singapore
Lindley PF (2001) In: Bertini I, Sigel A, Sigel H (eds) Multi-copper oxidases. Handbook on metalloproteins. Dekker, New York, pp 763–811
Xu F (1999) In: Flickinger MC, Drewn SW (eds) Encyclopedia of bioprocess technology: fermentation, biocatalysis and bioseparation. Wiley, New York, pp 1545–1554
Gianfreda L, Xu F, Bollag J-M (1999) Bioremediation J 3:1–25
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) J Biol Chem 277:18849–18859
Enguita FJ, Martins LO, Henriques AO, Carrondo MA (2003) J Biol Chem 278:19416–19425
Enguita FJ, Marçal D, Martins LO, Grenha R, Henriques AO, Lindley PF, Carrondo MA (2004) J Biol Chem 279:23472–23476
Bento I, Martins LO, Lopes GG, Arménia MA, Lindley PF (2005) Dalton Trans 21:3507–3513
Xu F (1996) Biochemistry 35:7608–7614
Solomon EI, Sundaram UM, Machonkin TE (1996) Chem Rev 96:2563–2605
Karlin S, Zhu SZY, Karlin KD (1997) Proc Natl Acad Sci USA 94:14225–14230
Otwinowski Z, Minor W (1997) In: Carter CW Jr, Sweet RM (eds) Methods in enzymology, macromolecular crystallography, vol 276. Academic, New York, pp 307–326
CCP4 (1994) Acta Crystallogr Sect D 50:760–763
Murshudov GN, Vagin AA, Lebedev A, Wilson KS, Dodson EJ (1999) Acta Crystallogr Sect D 55:247–255
Emsley P, Cowtan K (2004) Acta Crystallogr Sect D 60:2126–2132
Ramakrishnan C, Ramachandran GN (1965) Biophys J 5:909–933
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) J Appl Crystallogr 26:283–291
Schneider TR (2002) Acta Crystallogr Sect D 58:195–208
Bradford MM (1976) Anal Biochem 72:248–254
Eftink MR (1994) Biophys J 66:482–501
Monsellier E, Bedouelle H (2005) Protein Eng Des Sel 18:445–456
Brenner AJ, Harris ED (1995) Anal Biochem 226:80–84
Ramachandran GN, Sasisekharan V (1968) Adv Protein Chem 23:283–437
Ducros V, Brzozowski AM, Wilson KS, Ostergaard P, Schneider P, Svendson A, Davies GJ (2001) Acta Crystallogr Sect D 57:333–336
Hakulinen N, Kiiskinen LL, Kruus K, Saloheimo M, Paananen A, Koivula A, Rouvinen J (2002) Nat Struct Biol 9:601–605
Garavaglia S, Cambria MT, Miglio M, Ragusa S, Iacobazzi V, Palmieri F, D’Ambrosio C, Scaloni A, Rizzi M (2004) J Mol Biol 342:1519–1531
DeLano WL (2002) The PyMOL molecular graphics system. DeLano Scientific, San Carlos
Xu F, Berka RM, Wahleithner JA, Nelson BA, Shuster JR, Brown SH, Palmer AE, Solomon EI (1998) Biochem J 334:63–70
Xu F, Palmer AE, Yaver DS, Berka RM, Gambeta GA, Brown SH, Solomon EI (1999) J Biol Chem 274:12372–12375
Palmer AE, Randall DW, Xu F, Solomon EI (1999) J Am Chem Soc 121:7138–7149
Pascher T, Karlsson BG, Nordling M, Malmström BG, Vänngård T (1993) Eur J Biochem 212:289–296
Hall JF, Kanbi LD, Strange RW, Hasnain SS (1999) Biochemistry 38:12675–12680
Diederix REM, Canters GW, Dennison C (2000) Biochemistry 39:9551–9560
Kataoka K, Kitagawa R, Inoue M, Naruse D, Sakurai T, Huang H-W (2005) Biochemistry 44:7004–7012
Moser CC, Dutton PL (1996) In: Bendall DS (ed) Protein electron transfer. Bios Scientific, Oxford, pp 1–21
Warshel A (1978) Proc Natl Acad Sci USA 75:5250–5254
Lakowicz JR (1999) Principles of fluorescence spectroscopy. Kluwer/Plenum, New York
Kelly SM, Price NC (1997) Biochim Biophys Acta 1338:161–185
Bonomo RP, Cennamo G, Purrello R, Santoro AM, Zappala RJ (2001) Inorg Chem 83:67–75
Agostinelli E, Cervoni L, Giartosio A, Morpurgo L (1995) Biochem J 306:697–702
Koroleva OV, Stepanova EV, Binukov VI, Timofeev VP, Pfeil W (2001) Biochim Biophys Acta 1547:397–407
Fersht A (1999) Structure and mechanism in protein science. Freeman, New York
Acknowledgements
We would like to thank our colleagues at the Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa (ITQB-UNL), Cláudio M. Soares, António M. Baptista and Manuela M. Pereira for support and useful discussions. We would also like to thank João Carita for help with the cell growth in the Organic Fermentation Unit at the ITQB-UNL. The ITQB-UNL and the Fundação para a Ciência e a Tecnológia provided the resources necessary for this research. All X-ray data were collected at the European Synchrotron Radiation Facility, Grenoble, France, with the kind assistance of the scientists responsible for the operation of beam line ID29.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The thermodynamic stability of CotA wild type monitored by fluorescence was analysed according to a two-state process (N↔U) using the following equations:
and
where N and U are native and unfolded CotA, respectively, y is the fluorescence signal, f is the fraction of CotA molecules with a given conformation, K is the equilibrium constant, ΔG 0 is the standard free energy, m (U–N) is the linear dependence of ΔG 0 on GdnHCl concentration and [GdnHCl]50% is the GdnHCl concentration for ΔG 0=0. y N and y U were calculated directly from the pretransition and posttransition regions according to a linear dependence.
The thermodynamic stability evaluated by activity measurements and the absorbance at 600 nm are dependent on copper loss from T1. Therefore Eqs. 1, 2, 3, 4 and 5 were used but considering that the two-state process assessed by these two techniques is the establishment between the native state with copper bound at T1 and the native state with no copper at T1 (N↔N(no copper)).
The thermodynamic stability of CotA M502L and M502F mutants monitored by fluorescence could only be accurately fitted according to a three-state process, with the accumulation of an intermediate state in-between N and U (N↔I↔U). The following equations were used:
and
where y N was considered to be the fluorescence signal at 0 M GdnHCl (this assumption was confirmed by the fluorescence emission maximum) and y U was calculated directly from the posttransition regions according to a linear dependence.
Combining Eqs. 6, 7, 8, 9, 10, 11 and 12, we fitted the fluorescence signal (and therefore fractional change of fluorescence signal) according to Eq. 13:
The fits were carried out by varying values of y I, ΔG 0 water(I–N) , m (I–N), ΔG 0 water(U–I) and m (U–I) with the Origin software using the non-linear curve-fit option. Combination of Eqs. 6, 7 and 8 leads to
and
Rights and permissions
About this article
Cite this article
Durão, P., Bento, I., Fernandes, A.T. et al. Perturbations of the T1 copper site in the CotA laccase from Bacillus subtilis: structural, biochemical, enzymatic and stability studies. J Biol Inorg Chem 11, 514–526 (2006). https://doi.org/10.1007/s00775-006-0102-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-006-0102-0