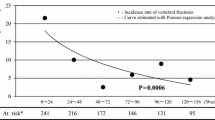
Abstract
Factors associated with an inadequate response (IR) to bisphosphonates have been reported in many countries, but not in Japan, where the approved dose is half the global dose. We analyzed factors associated with IR to risedronate in Japanese patients with osteoporosis. This was a post hoc analysis of 1261 Japanese osteoporosis patients who received risedronate for 1 year in phase III trials. IR was defined as more than one new vertebral fracture (VF) and/or negative change in lumbar spine bone mineral density (BMD) at 1 year. Various baseline and follow-up variables were examined for potential contribution to IR. Of the 1261 subjects, 118 exhibited an IR. At baseline, IR was associated with a higher BMD, lower levels of bone turnover markers (BTM) (serum bone-specific alkaline phosphatase, urinary N-terminal telopeptide of type 1 collagen and C-terminal telopeptide of type 1 collagen), and serum 25-hydroxyvitamin D [25(OH)D] below 16 ng/mL. BTM changes were blunted at 6 months in subjects with IR. On simple regression analysis, all the above variables and poor drug adherence were associated with an IR. On multivariate regression analysis, factors associated with IR were high BMD, vitamin D deficiency at baseline and low BTM at baseline, or a decreased BTM response at 6 months. Low serum 25(OH)D and BTM as well as high BMD at baseline were independent predictors of an IR to risedronate in Japan. These results emphasize the importance of the assessment of serum 25(OH)D and BTM in the management of osteoporosis with bisphosphonates.

Similar content being viewed by others
References
Cranney A, Tugwell P, Adachi J, Weaver B, Zytaruk N, Papaioannou A, Robinson V, Shea B, Wells G, Guyatt G (2002) Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 23:517–523. https://doi.org/10.1210/er.2001-3002
Heckman GA, Papaioannou A, Sebaldt RJ, Ioannidis G, Petrie A, Goldsmith C, Adachi JD (2002) Effect of vitamin D on bone mineral density of elderly patients with osteoporosis responding poorly to bisphosphonates. BMC Musculoskelet Disord 3:6
Adami S, Isaia G, Luisetto G, Minisola S, Sinigaglia L, Gentilella R, Agnusdei D, Iori N, Nuti R, ICARO Study Group (2006) Fracture incidence and characterization in patients on osteoporosis treatment: the ICARO study. J Bone Miner Res 21:1565–1570. https://doi.org/10.1359/jbmr.060715
Rabenda V, Mertens R, Fabri V, Vanoverloop J, Sumkay F, Vannecke C, Deswaef A, Verpooten GA, Reginster JY (2008) Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int 19:811–818. https://doi.org/10.1007/s00198-007-0506-x
Carmel AS, Shieh A, Bang H, Bockman RS (2012) The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥ 33 ng/ml. Osteoporos Int 23:2479–2487. https://doi.org/10.1007/s00198-011-1868-7
Díez-Pérez A, Olmos JM, Nogués X, Sosa M, Díaz-Curiel M, Pérez-Castrillón JL, Pérez-Cano R, Muñoz-Torres M, Torrijos A, Jodar E, Del Rio L, Caeiro-Rey JR, Farrerons J, Vila J, Arnaud C, González-Macías J (2012) Risk factors for prediction of inadequate response to antiresorptives. J Bone Miner Res 27:817–824. https://doi.org/10.1002/jbmr.1496
Cairoli E, Eller-Vainicher C, Ulivieri FM, Zhukouskaya VV, Palmieri S, Morelli V, Beck-Peccoz P, Chiodini I (2014) Factors associated with bisphosphonate treatment failure in postmenopausal women with primary osteoporosis. Osteoporos Int 25:1401–1410. https://doi.org/10.1007/s00198-014-2619-3
Díez-Pérez A, Adachi JD, Adami S, Anderson FA Jr, Boonen S, Chapurlat R, Compston JE, Cooper C, Gehlbach SH, Greenspan SL, Hooven FH, LaCroix AZ, Nieves JW, Netelenbos JC, Pfeilschifter J, Rossini M, Roux C, Saag KG, Silverman S, Siris ES, Wyman A, Rushton-Smith SK, Watts NB; Global Longitudinal Study of Osteoporosis in Women GLOW Investigators (2014) Risk factors for treatment failure with antiosteoporosis medication: the global longitudinal study of osteoporosis in women (GLOW). J Bone Miner Res 29:260–267. https://doi.org/10.1002/jbmr.2023
Hawley S, Javaid MK, Rubin KH, Judge A, Arden NK, Vestergaard P, Eastell R, Díez-Pérez A, Cooper C, Abrahamsen B, Prieto-Alhambra D (2016) Incidence and predictors of multiple fractures despite high adherence to oral bisphosphonates: a binational population-based cohort study. J Bone Miner Res 31:234–244. https://doi.org/10.1002/jbmr.2595
Burnett-Bowie SM, Saag K, Sebba A, de Papp AE, Chen E, Rosenberg E, Greenspan SL (2009) Prediction of changes in bone mineral density in postmenopausal women treated with once-weekly bisphosphonates. J Clin Endocrinol Metab 94:1097–1103. https://doi.org/10.1210/jc.2008-1122
Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY (2000) Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab 85:1895–1900. https://doi.org/10.1210/jcem.85.5.6603
Seibel MJ, Naganathan V, Barton I, Grauer A (2004) Relationship between pretreatment bone resorption and vertebral fracture incidence in postmenopausal osteoporotic women treated with risedronate. J Bone Miner Res 19:323–329. https://doi.org/10.1359/jbmr.0301231
Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151. https://doi.org/10.1359/jbmr.040920
Sebba AI (2008) Significance of a decline in bone mineral density while receiving oral bisphosphonate treatment. Clin Ther 30:443–452. https://doi.org/10.1016/j.clinthera.2008.03.008
Bonnick SL (2000) Monitoring osteoporosis therapy with bone densitometry: a vital tool or regression toward mediocrity? J Clin Endocrinol Metab 85:3493–3495. https://doi.org/10.1210/jcem.85.10.6960
Cummings SR, Palermo L, Browner W, Marcus R, Wallace R, Pearson J, Blackwell T, Eckert S, Black D (2000) Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research Group. JAMA 283:1318–1321
Watts NB, Lewiecki EM, Bonnick SL, Laster AJ, Binkley N, Blank RD, Geusens PP, Miller PD, Petak SM, Recker RR, Saag KG, Schousboe J, Siris ES, Bilezikian JP (2009) Clinical value of monitoring BMD in patients treated with bisphosphonates for osteoporosis. J Bone Miner Res 24:1643–1646. https://doi.org/10.1359/jbmr.090818
Watts NB, Cooper C, Lindsay R, Eastell R, Manhart MD, Barton IP, van Staa TP, Adachi JD (2004) Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom 7:255–261
Miller PD, Delmas PD, Huss H, Patel KM, Schimmer RC, Adami S, Recker RR (2010) Increases in hip and spine bone mineral density are predictive for vertebral antifracture efficacy with ibandronate. Calcif Tissue Int 87:305–313. https://doi.org/10.1007/s00223-010-9403-y
Hagino H, Yoshida S, Hashimoto J, Matsunaga M, Tobinai M, Nakamura T (2014) Increased bone mineral density with monthly intravenous ibandronate contributes to fracture risk reduction in patients with primary osteoporosis: three-year analysis of the MOVER study. Calcif Tissue Int 95:557–563. https://doi.org/10.1007/s00223-014-9927-7
Leslie WD, Majumdar SR, Morin SN, Lix LM (2016) Change in bone mineral density is an indicator of treatment-related antifracture effect in routine clinical practice: a registry-based cohort study. Ann Intern Med 165:465–472. https://doi.org/10.7326/m15-2937
Inoue D, Muraoka R, Okazaki R, Nishizawa Y, Sugimoto T (2016) Efficacy and safety of risedronate in osteoporosis subjects with comorbid diabetes, hypertension, and/or dyslipidemia: a post hoc analysis of phase III trials conducted in Japan. Calcif Tissue Int 98:114–122. https://doi.org/10.1007/s00223-015-0071-9
Mawatari T, Muraoka R, Iwamoto Y (2017) Relationship between baseline characteristics and response to risedronate treatment for osteoporosis: data from three Japanese phase III trials. Osteoporos Int 28:1279–1286. https://doi.org/10.1007/s00198-016-3848-4
Kishimoto H, Fukunaga M, Kushida K, Shiraki M, Itabashi A, Nawata H, Nakamura T, Ohta H, Takaoka K, Ohashi Y (2006) Efficacy and tolerability of once-weekly administration of 17.5 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with 2.5-mg once-daily dosage regimen. J Bone Miner Metab 24:405–413. https://doi.org/10.1007/s00774-006-0706-z
Hagino H, Kishimoto H, Ohishi H, Horii S, Nakamura T (2014) Efficacy, tolerability and safety of once-monthly administration of 75 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with a 2.5 mg once-daily dosage regimen. Bone 59:44–52. https://doi.org/10.1016/j.bone.2013.10.017
Ouchi Y, Orimo H (1989) The disease of the old age and metabolic change of calcium and magnesium (in Japanese). Jpn J Geriat 26:216–221
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 19:331–337
Office of Freedom of Information (1994) Guidelines for preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis. Department of Health and Human Services, Rockville, MD, USA
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Min Res 8:1137–1148
Nishizawa Y, Ohta H, Miura M, Inaba M, Ichimura S, Shiraki M, Takada J, Chaki O, Hagino H, Fujiwara S, Fukunaga M, Miki T, Yoshimura N (2013) Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 edition). J Bone Miner Metab 31:1–15. https://doi.org/10.1007/s00774-012-0392-y
Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, Eastell R, Eriksen EF, Gonzalez-Macias J, Liberman UA, Wahl DA, Seeman E, Kanis JA, Cooper C; IOF CSA Inadequate Responders Working Group (2012) Treatment failure in osteoporosis. Osteoporos Int 23:2769–2774. https://doi.org/10.1007/s00198-012-2093-8
Sawka AM, Adachi JD, Ioannidis G, Olszynski WP, Brown JP, Hanley DA, Murray T, Josse R, Sebaldt RJ, Petrie A, Tenenhouse A, Papaioannou A, Goldsmith CH (2003) What predicts early fracture or bone loss on bisphosphonate therapy? J Clin Densitom 6:315–322
Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB (2011) Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res 26:1662–1669. https://doi.org/10.1002/jbmr.342
Hochberg MC, Ross PD, Black D, Cummings SR, Genant HK, Nevitt MC, Barrett-Connor E, Musliner T, Thompson D (1999) Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthr Rheum 42:1246–1254. https://doi.org/10.1002/1529-0131(199906)42:6<1246::AID-ANR22>3.0.CO;2-U
Lewiecki EM (2003) Nonresponders to osteoporosis therapy. J Clin Densitom 6(4):307–314
Okazaki R, Ozono K, Fukumoto S, Inoue D, Yamauchi M, Minagawa M, Michigami T, Takeuchi Y, Matsumoto T, Sugimoto T (2016) Assessment criteria for vitamin D deficiency/insufficiency in Japan: proposal by an expert panel supported by the Research Program of Intractable Diseases, Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research and the Japan Endocrine Society [Opinion]. J Bone Miner Metab. https://doi.org/10.1007/s00774-016-0805-4
Uchida S, Taniguchi T, Shimizu T, Kakikawa T, Okuyama K, Okaniwa M, Arizono H, Nagata K, Santora AC, Shiraki M, Fukunaga M, Tomomitsu T, Ohashi Y, Nakamura T (2005) Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab 23:382–388. https://doi.org/10.1007/s00774-005-0616-5
Nakamura T, Ito M, Hashimoto J, Shinomiya K, Asao Y, Katsumata K, Hagino H, Inoue T, Nakano T, Mizunuma H (2015) Clinical efficacy and safety of monthly oral ibandronate 100 mg versus monthly intravenous ibandronate 1 mg in Japanese patients with primary osteoporosis. Osteoporos Int 26:2685–2693. https://doi.org/10.1007/s00198-015-3175-1
Okazaki R, Hagino H, Ito M, Sone T, Nakamura T, Mizunuma H, Fukunaga M, Shiraki M, Nishizawa Y, Ohashi Y, Matsumoto T (2012) Efficacy and safety of monthly oral minodronate in patients with involutional osteoporosis. Osteoporos Int 23:1737–1745. https://doi.org/10.1007/s00198-011-1782-z
Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Morii H, Ohashi Y, Nakamura T (2009) Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int 20:1429–1437. https://doi.org/10.1007/s00198-008-0816-7
Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, Kaneda K, Minaguchi H, Inoue T, Morii H, Tomita A, Yamamoto K, Nagata Y, Nakashima M, Orimo H (1999) A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The Alendronate Phase III Osteoporosis Treatment Research Group. Osteoporos Int 10:183–192
Fukunaga M, Kushida K, Kishimoto H, Shiraki M, Taketani Y, Minaguchi H, Inoue T, Morita R, Morii H, Yamamoto K, Ohashi Y, Orimo H (2002) A comparison of the effect of risedronate and etidronate on lumbar bone mineral density in Japanese patients with osteoporosis: a randomized controlled trial. Osteoporos Int 13:971–979. https://doi.org/10.1007/s001980200135
Fujita T, Orimo H, Inoue T, Kaneda K, Sakurai M, Morita R, Morii H, Yamamoto K, Sugioka Y, Inoue A, Hoshino Y, Kawaguchi H, Yamamoto I, Fukase M, Takaoka K (1993) Double-blind mulitcenter comparative study with alphacalcidol of etidronate disodium(EHDP) in involutional osteoporosis. Clin Eval 21:261–302 (in Japanese)
Civitelli R, Gonnelli S, Zacchei F, Bigazzi S, Vattimo A, Avioli LV, Gennari C (1988) Bone turnover in postmenopausal osteoporosis. Effect of calcitonin treatment. J Clin Invest 82:1268–1274. https://doi.org/10.1172/jci113725
Gonnelli S, Cepollaro C, Pondrelli C, Martini S, Monaco R, Gennari C (1997) The usefulness of bone turnover in predicting the response to transdermal estrogen therapy in postmenopausal osteoporosis. J Bone Miner Res 12:624–631. https://doi.org/10.1359/jbmr.1997.12.4.624
Bauer DC, Garnero P, Hochberg MC, Santora A, Delmas P, Ewing SK, Black DM (2006) Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 21:292–299. https://doi.org/10.1359/jbmr.051018
Johansson H, Oden A, Kanis JA, McCloskey EV, Morris HA, Cooper C, Vasikaran S (2014) A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif Tissue Int 94:560–567. https://doi.org/10.1007/s00223-014-9842-y
Naylor KE, Jacques RM, Paggiosi M, Gossiel F, Peel NF, McCloskey EV, Walsh JS, Eastell R (2016) Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 27:21–31. https://doi.org/10.1007/s00198-015-3145-7
Gordon MS, Gordon MB (2002) Response of bone mineral density to once-weekly administration of risedronate. Endocr Pract 8:202–207. https://doi.org/10.4158/ep.8.3.202
Majima T, Shimatsu A, Komatsu Y, Satoh N, Fukao A, Ninomiya K, Matsumura T, Nakao K (2008) Association between baseline values of bone turnover markers and bone mineral density and their response to raloxifene treatment in Japanese postmenopausal women with osteoporosis. Endocr J 55:41–48
Niimi R, Kono T, Nishihara A, Hasegawa M, Matsumine A, Kono T, Sudo A (2014) Determinants associated with bone mineral density increase in response to daily teriparatide treatment in patients with osteoporosis. Bone 66:26–30. https://doi.org/10.1016/j.bone.2014.05.017
Kishimoto H, Maehara M (2015) Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos 10:231. https://doi.org/10.1007/s11657-015-0231-6
Acknowledgements
This study was supported by the Joint Development Program of EA Pharma Co., Ltd. and Takeda Pharmaceutical Co., Ltd. Funding for writing and editorial support was provided by EA Pharma Co., Ltd. This study was in part supported by the Research Program of Intractable Diseases provided by the Ministry of Health, Labour and Welfare of Japan. Pacific Edit reviewed the manuscript prior to submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Ryo Okazaki received grants from Asahi-Kasei Pharma, Astellas Pharma, Chugai Pharmaceutical, Daiichi-Sankyo, EA Pharma, Eisai, Eli Lilly Japan, Ono Pharmaceutical, Taisho-Toyama Pharmaceutical, Takeda Pharmaceutical, and Teijin Pharma, outside of the submitted work. Mr. Ryoichi Muraoka and Mr. Masayuki Maehara received personal fees from EA Pharma Co. Ltd., during the course of the study. Dr. Daisuke Inoue received personal fees from EA Pharma, grants from Asahi-Kasei Pharma, Astellas Pharma, Chugai Pharmaceutical, Daiichi-Sankyo, Eisai, Eli Lilly Japan, Pfizer, Ono Pharmaceutical, Taisho-Toyama Pharmaceutical, Takeda Pharmaceutical, and Teijin Pharma, outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
About this article
Cite this article
Okazaki, R., Muraoka, R., Maehara, M. et al. Factors associated with inadequate responses to risedronate in Japanese patients with osteoporosis. J Bone Miner Metab 37, 185–197 (2019). https://doi.org/10.1007/s00774-018-0931-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-018-0931-2