Abstract
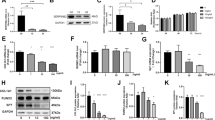
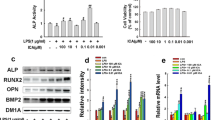
Phosphorylation of eukaryotic initiation factor 2α (eIF2α), transiently activated by various cellular stresses, is known to alleviate stress-induced cellular damage. Here, we addressed a question: does elevation of eIF2α phosphorylation by salubrinal (a pharmacological inhibitor of eIF2α dephosphorylation) enhance healing of bone wounds? We hypothesized that salubrinal would accelerate a closure of surgically generated bone holes by modifying expression of stress-sensitive genes. To examine this hypothesis, we employed a rat wound model. Surgical wounds were generated on anterior and posterior femoral cortexes, and salubrinal was locally administered on the anterior side. The results showed that, compared to a contralateral control, the size of surgical wounds was reduced by 10.8 % (day 10) and 18.0 % (day 20) on the anterior side (both p < 0.001), and 4.1 % (day 10; p < 0.05) and 11.1 % (day 20; p < 0.001) on the posterior side. In addition, salubrinal locally elevated cortical thickness and increased BMD and BMC. Pharmacokinetic analysis revealed that subcutaneous injection of salubrinal transiently increased its concentration in plasma followed by a rapid decrease within 24 h, and its half-life in plasma was 1.2 h. Salubrinal altered the phosphorylation level of eIF2α as well as the mRNA levels of ATF3, ATF4, and CHOP, and suppressed cell death induced by stress to the endoplasmic reticulum. In summary, the results herein demonstrate that subcutaneous administration of salubrinal accelerates healing of surgically generated bone holes through the modulation of eIF2α phosphorylation.








Similar content being viewed by others
References
Harding HP, Calfon M, Urano F, Novoa I, Ron D (2002) Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Dev Biol 18:575–599
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7:880–885
Wek RC, Cavener DR (2007) Translational control and the unfolded protein response. Antioxid Redox Signal 9:2357–2371
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Invest 110:1383–1388
Boyce M, Yuan J (2006) Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ 13:363–373
Ron D, Harding HP (2007) eIF2α phosphorylation in cellular stress responses and disease. Transl Control Biol Med 13:349–372
Proud CG (2005) eIF2 and the control of cell physiology. Semin Cell Dev Biol 16:3–12
Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101:11269–11274
Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G (2004) ATF4 is a substrate of RSK2 and an essential regulator of osteoblasts biology: implication for Coffin–Lowry syndrome. Cell 117:387–398
Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307:935–939
Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korthonen L (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 27:901–908
Zhu Y, Fenik P, Zhan G, Sanfillipo-Cohn B, Naidoo N, Veasey SC (2008) eIF-2α protects brainstem motoneurons in a murine model of sleep apnea. J Neurosci 28:2168–2178
Zhang P, Sun Q, Turner CH, Yokota H (2007) Knee loading accelerates bone healing in mice. J Bone Miner Res 22:1979–1987
Wang JW, Xu SW, Yang DS, Lv RK (2007) Locally applied simvastatin promotes fracture healing in ovariectomized rat. Osteoporos Int 18:1641–1650
Zhang P, Yokota H (2007) Effects of surgical holes in mouse tibiae on bone formation induced by knee loading. Bone (NY) 40:1320–1328
Zhang P, Tanaka SM, Jiang H, Su M, Yokota H (2006) Diaphyseal bone formation in murine tibiae in response to knee loading. J Appl Physiol 100:1452–1459
Zhang P, Tanaka S, Sun Q, Turner CH, Yokota H (2007) Frequency-dependent enhancement of bone formation in murine tibiae and femora with knee loading. J Bone Miner Metab 25:383–391
Zhang P, Su M, Tanaka S, Yokota H (2006) Knee loading causes diaphyseal cortical bone formation in murine femurs. BMC Musculoskelet Dis 73:1–12
Zhang P, Turner CH, Yokota H (2009) Joint loading-driven bone formation and signaling pathways predicted from genome-wide expression profiles. Bone (NY) 44:989–998
Chiba S, Okada K, Lee K, Segre GV, Neer RM (2001) Molecular analysis of defect healing in rat diaphyseal bone. J Vet Med Sci 63:603–606
Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT (1999) Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res 14:893–903
Uchida S, Sakai A, Kudo H, Otomo H, Watanuki M, Tanaka M, Nagashima M, Nakamura T (2003) Vascular endothelial growth factor is expressed along with its receptors during the healing process of bone and bone marrow after drill-hole injury in rats. Bone (NY) 32:491–501
Chen H, Sun J, Hoemann CD, Lascau-Coman V, Ouyang W, McKee MD, Shive MS, Buschmann MD (2009) Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res 27:1432–1438
Li G, White G, Connolly C, March D (2002) Cell proliferation and apoptosis during fracture healing. J Bone Miner Res 17:791–799
Hirasawa H, Jiang C, Zhang P, Yang FC, Yokota H (2010) Mechanical stimulation suppresses phosphorylation of eIF2α and PERK-mediated responses to stress to the endoplasmic reticulum. FEBS Lett 584:745–752
Zhang P, Yokota H (2009) Salubrinal stimulates anabolic responses in mouse femora. 55th Annual Meeting of the Orthopaedic Research Society, Las Vegas, USA
Wu J, Kaufman RJ (2006) From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13:374–384
Hamamura K, Yokota H (2007) Stress to endoplasmic reticulum of mouse osteoblasts induces apoptosis and transcriptional activation for bone remodeling. FEBS Lett 581:1769–1774
Chen A, Hamamura K, Zhang P, Chen Y, Yokota H (2010) Systems analysis of bone remodeling as a homeostatic regulator. IET Syst Biol 4:52–63
James CG, Woods A, Underhill TM, Beier F (2006) The transcription factor ATF3 is upregulated during chondrocyte differentiation and represses cyclin D1 and A gene transcription. BMC Mol Biol 30:1–11
Su N, Kilberg MS (2008) C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem 283:35106–35117
Pereira RC, Stadmeyer L, Marciniak SJ, Ron D, Canalis E (2006) C/EBP homologous protein is necessary for normal osteoblastic function. J Cell Biochem 97:633–640
Long K, Boyce M, Lin H, Yuan J, Ma D (2005) Structure–activity relationship studies of salubrinal lead to its active biotinylated derivative. Bioorg Med Chem 15:3849–3852
Acknowledgments
This work was in part supported by DOD Congressionally Directed Medical Research Program PR100092 (HY), NIH R03AR55322 (PZ), and NIH R01AR52144 (HY).
Conflict of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zhang, P., Hamamura, K., Jiang, C. et al. Salubrinal promotes healing of surgical wounds in rat femurs. J Bone Miner Metab 30, 568–579 (2012). https://doi.org/10.1007/s00774-012-0359-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-012-0359-z