Abstract
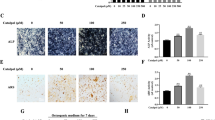
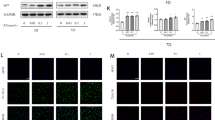
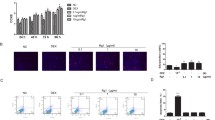
Salidroside (SAL), a major active component of Rhodiola rosea L., exhibits diverse pharmacological effects. However, the direct roles of SAL in fracture healing remain largely unknown. Here, we demonstrate that SAL significantly promotes proliferation by altering the cell-cycle distribution of osteoblastic cells. SAL also greatly stimulates osteoblast differentiation and mineralization by inducing the expression of Runx2 and Osterix. In addition to its osteoblast-autonomous effects, SAL can activate the HIF-1α pathway coupling of angiogenesis and osteogenesis through cell-non-autonomous effects. Our in vitro results suggest that SAL significantly up-regulates HIF-1α expression at the mRNA and protein levels. Furthermore, the nuclear translocation and transcriptional activity of HIF-1α and the HIF-responsive gene VEGF increase following SAL treatment. Our mechanistic study revealed that the regulation of osteoblastic proliferation and HIF-1α expression partly involves MAPK/ERK and PI3K/Akt signaling. Our in vivo analysis also demonstrated that SAL can promote angiogenesis within the callus and accelerate fracture healing. Thus, SAL promotes skeletal regeneration in cell-autonomous and cell-non-autonomous ways and might be a potential therapy for accelerating fracture healing.









Similar content being viewed by others
References
Artigas N, Ureña C, Rodríguez-Carballo E, Rosa JL, Ventura F (2014) Mitogen-activated protein kinase (MAPK)-regulated interactions between Osterix and Runx2 are critical for the transcriptional osteogenic program. J Biol Chem 289:27105–27117
Blouin CC, Pagé EL, Soucy GM, Richard DE (2004) Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood 103:1124–1130
Carragee EJ, Hurwitz EL, Weiner BK, Bono CM, Rothman DJ (2011) Future directions for the spine journal: managing and reporting conflict of interest issues. Spine J 11:695–697
Chen JJ, Zhang NF, Mao GX, He XB, Zhan YC, Deng HB, Song DQ, Li DD, Li ZR, Si SY, Qiu Q, Wang Z (2013) Salidroside stimulates osteoblast differentiation through BMP signaling pathway. Food Chem Toxicol 62:499–505
Choi IH, Ahn JH, Chung CY, Cho TJ (2000) Vascular proliferation and blood supply during distraction osteogenesis: a scanning electron microscopic observation. J Orthop Res 18:698–705
Choi P, Ogilvie C, Thompson Z, Miclau T, Helms JA (2004) Cellular and molecular characterization of a murine non-union model. J Orthop Res 22:1100–1107
Colnot CI, Helms JA (2001) A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev 100:245–250
Coulibaly MO, Sietsema DL, Burgers TA, Mason J, Williams BO, Jones CB (2010) Recent advances in the use of serological bone formation markers to monitor callus development and fracture healing. Crit Rev Eukaryot Gene Expr 20:105–127
Court-Brown CM, McQueen MM (2008) Nonunions of the proximal humerus: their prevalence and functional outcome. J Trauma 64:1517–1521
Déry MA, Michaud MD, Richard DE (2005) Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol 37:535–540
Dimitriou R, Jones E, McGonagle D, Giannoudis PV (2011) Bone regeneration: current concepts and future directions. BMC Med 9:66
Donneys A, Ahsan S, Perosky JE, Deshpande SS, Tchanque-Fossuo CN, Levi B, Kozloff KM, Buchman SR (2013) Deferoxamine restores callus size, mineralization and mechanical strength in fracture healing after radiotherapy. Plast Reconstr Surg 131:711e–719e
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754
Eckardt H, Ding M, Lind M, Hansen ES, Christensen KS, Hvid I (2005) Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J Bone Joint Surg (Br) 87:1434–1438
Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL (2002) Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem 277:38205–38211
Geiger F, Bertram H, Berger I, Lorenz H, Wall O, Eckhardt C, Simank HG, Richter W (2005) Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res 20:2028–2035
Hagiwara H, Inoue A, Yamaguchi A, Yokose S, Furuya M, Tanaka S, Hirose S (1996) cGMP produced in response to ANP and CNP regulates proliferation and differentiation of osteoblastic cells. Am J Physiol 270:C1311–C1318
Hiltunen A, Aro HT, Vuorio E (1993) Regulation of extracellular matrix genes during fracture healing in mice. Clin Orthop Relat Res 297:23–27
Hu X, Zhang X, Qiu S, Yu D, Lin S (2010) Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells. Biochem Biophys Res Commun 398:62–67
Karsenty G, Kronenberg HM, Settembre C (2009) Genetic control of bone formation. Annu Rev Cell Dev Biol 25:629–648
Komatsu DE, Hadjiargyrou M (2004) Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone 34:680–688
Krane SM (2005) Identifying genes that regulate bone remodeling as potential therapeutic targets. J Exp Med 201:841–843
Lee SH, Che X, Jeong JH, Choi JY, Lee YJ, Lee YH, Bae SC, Lee YM (2012) Runx2 protein stabilizes hypoxia-inducible factor-1alpha through competition with von Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth plate hypertrophic chondrocytes. J Biol Chem 287:14760–14771
Li HZ, Wang Y, Gao Y, Shao J, Zhao XL, Deng WM, Liu YX, Yang J, Yao Z (2008) Effects of raf kinase inhibitor protein expression on metastasis and progression of human epithelial ovarian cancer. Mol Cancer Res 6:917–928
Li L, Yin X, Ma N, Lin F, Kong X, Chi J, Feng Z (2014) Desferrioxamine regulates HIF-1 alpha expression in neonatal rat brain after hypoxia-ischemia. Am J Transl Res 6:377–383
Mangiavini L, Merceron C, Araldi E, Khatri R, Gerard-O’Riley R, Wilson TL, Rankin EB, Giaccia AJ, Schipani E (2014) Loss of VHL in mesenchymal progenitors of the limb bud alters multiple steps of endochondral bone development. Dev Biol 393:124–136
Mao GX, Deng HB, Yuan LG, Li DD, Li YY, Wang Z (2010) Protective role of salidroside against aging in a mouse model induced by D-galactose. Biomed Environ Sci 23:161–166
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29
Nishimura R, Wakabayashi M, Hata K, Matsubara T, Honma S, Wakisaka S, Kiyonari H, Shioi G, Yamaguchi A, Tsumaki N, Akiyama H, Yoneda T (2012) Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J Biol Chem 287:33179–33190
Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol 2:423–427
Ortuño MJ, Susperregui AR, Artigas N, Rosa JL, Ventura F (2013) Osterix induces Col1a1 gene expression through binding to Sp1 sites in the bone enhancer and proximal promoter regions. Bone 52:548–556
Pagé EL, Robitaille GA, Pouysségur J, Richard DE (2002) Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem 277:48403–48409
Parker MJ, Raghavan R, Gurusamy K (2007) Incidence of fracture-healing complications after femoral neck fractures. Clin Orthop Relat Res 458:175–179
Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, Huard J (2005) VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res 20:2017–2027
Ryoo HM, Lee MH, Kim YJ (2006) Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene 366:51–57
Salceda S, Caro J (1997) Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 272:22642–22647
Stains JP, Civitelli R (2003) Genomic approaches to identifying transcriptional regulators of osteoblast differentiation. Genome Biol 4:222
Street J, Bao M, deGuzman L, Bunting S, Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A 99:9656–9661
Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, Clemens TL (2008) Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A 105:686–691
Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL (2007) The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117:1616–1626
Yu P, Hu C, Meehan EJ, Chen L (2007) X-ray crystal structure and antioxidant activity of salidroside, a phenylethanoid glycoside. Chem Biodivers 4:508–513
Zhang J, Liu A, Hou R, Zhang J, Jia X, Jiang W, Chen J (2009) Salidroside protects cardiomyocyte against hypoxia-induced death: a HIF-1alpha-activated and VEGF-mediated pathway. Eur J Pharmacol 607:6–14
Zhang JK, Yang L, Meng GL, Yuan Z, Fan J, Li D, Chen JZ, Shi TY, Hu HM, Wei BY, Luo ZJ, Liu J (2013) Protection by salidroside against bone loss via inhibition of oxidative stress and bone-resorbing mediators. PLoS ONE 8:e57251
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (nos. 81273520, 81572852, and 81502256), the Great Program of the Science Foundation of Tianjin (nos. 12JCZDJC26300 and 15JCYBJC28900) and the Great Program for Science and Technology in Logistics College of Chinese People’s Armed Police Forces (nos. WHZ201202, WHB201404, WHB201405 and WHB201406).
Author contributions
Y.W. and Z.M.H. designed the experiments. X.Q.G. and J.Y. analyzed the data and wrote the manuscript. L.Q., C.W. and Y.Q. performed the in vitro experiments. X.Q.G. and L.L. prepared the figures. Z.M.L. and D.W. performed the animal experiments. All of the authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
All animal procedures were approved by the Ethics Committee of Logistics College of Chinese People’s Armed Police Forces. The care, use and treatment of mice were in compliance with the guidelines of the Care and Use of Laboratory Animals.
Additional information
Xiao Qin Guo, Lin Qi and Jing Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, X.Q., Qi, L., Yang, J. et al. Salidroside accelerates fracture healing through cell-autonomous and non-autonomous effects on osteoblasts. Cell Tissue Res 367, 197–211 (2017). https://doi.org/10.1007/s00441-016-2535-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2535-2