Abstract
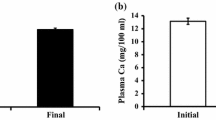
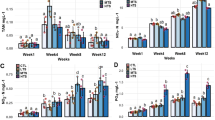
Silicon has been known as an essential element for bone formation. The silicon contents of sea water increase with increasing of depth: 1.8 ppm Si in deep-sea water (DW) at 612 m in depth versus 0.06 ppm in surface sea water (SW). The effects of soluble silicon (Si) and DW from which NaCl was eliminated were studied in comparison with tap water (TW) and SW in cell cultures and in animal experiments using the control strain of senescence accelerated mouse, SAMR1. Si at 10 ppm as sodium metasilicate or 10% DW in the α-MEM medium stimulated cellular viability, marker enzymes of osteoblast and osteoclast cell lines, and the 45CaCl2 uptake in those cells in comparison with the medium control. After weanling SAMR1 were maintained for 6 months on a diet containing 200 ppm Si and 39% of DW and SW, DW and Si improved bone biochemical indices such as femoral weight, mineral and collagen content, and marker enzymes of bone formation and resorption as well as mechanical properties as compared to TW. In the femoral bone marrow of SAMR1, the mRNA expression of bone morphogenetic protein-2 (BMP-2), interleukin-11 (IL-11), and runt-related transcription factor 2 (Runx 2), which stimulate osteoblast development as well as type I procollagen (COL1A1) mRNA, were significantly increased in both DW and Si groups. The expressions of both osteoprotegerin (OPG) and receptor activator of NF-κB ligand (RANKL) were also elevated, resulting in distinct increases of the OPG/RANKL ratio in both DW and Si groups. The results indicated that a soluble silicate and deep-sea water as its natural material stimulated cell growth in both osteoblasts and osteoclasts in cell culture and promoted bone metabolic turnover in favor of bone formation through stimulation of the related mRNA expression in animal experiments.
Similar content being viewed by others
References
Saltman PD, Strause LG (1993) The role of trace minerals in osteoporosis. J Am Coll Nutr 12:384–389
Carlisle EM (1972) Silicon: an essential element for the chick. Science 178:619–621
Schwarz K, Milne DB (1972) Growth-promoting effects of silicon in rats. Nature (Lond) 239:333–334
Carlisle EM (1984) Silicon. In: Frieden E (ed) Biochemistry of the Essential Ultratrace Elements. Plenum Press, New York, pp 257–291
LeVier RR (1975) Distribution of silicon in the adult rat and rhesus monkey. Bioinorg Chem 4:109–115
Charnot Y, Peres G (1978) Silicon, endocrine balance and mineral metabolism. In: Bendz G, Linquist I (eds) Biochemistry of Silicon and Related Problems. Plenum Press, New York, pp 269–279
Hott M, de Pollak C, Modrowski D, Marie PJ (1993) Short-time effects of organic silicon on trabecular bone in mature ovariectomized rats. Calcif Tissue Int 53:174–179
Rico H, Gallego-Lago JL, Hernandez ER, Villa LF, Sanchez-Atrio A, Seco C, Gervas JJ (2000) Effect of silicon supplement on osteopenia induced by ovariectomy in rats. Calcif Tissue Int 66:53–55
D’Haese PC, Shaheen FA, Huraib SO, Djukanovic L, Polenakovic MH, Spasovski G, Shikole A, Schurgers ML, Daneels RF, Lamberts LV, Van Landeghem GF, De Broe ME (1995) Increased silicon levels in dialysis patients due to high silicon content in the drinking water, inadequate water treatment procedures, and concentrate contamination: a multicentre study. Nephrol Dial Transplant 10:1838–1844
Poppwell JF, King SJ, Day JP, Ackrill P, Fifield LK, Cresswell RG, di Tada ML, Liu K (1998) Kinetics of uptake and elimination of silicic acid by a human subject: A novel application of 32Si and accelerator mass spectorometry. J Inorg Biochem 69:177–180
Reffitt DM, Jugdaohsingh R, Thompson RPH, Powell JJ (1999) Silicic acid: its gastrointestinal uptake and urinary excretion in man and effects on aluminium excretion. J Inorg Biochem 76:141–147
Broecker WS, Peng TH (1982) Tracers in the Sea. The Lamont-Doherty Geological Observatory, Columbia University, New York, pp 1–44
Roles OA, Laurence S, Van Hemelryck L (1979) The utilization of cold, nutrient-rich deep ocean water for energy and mariculture. Ocean Manag 5:199–210
Reffitt DM, Osgton N, Jugdaohsingh R, Cheung HFJ, Thompson RPH, Powell JJ, Hampson GN (2003) Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone (NY) 32:127–135
Tarutani T (1989) Polymerization of silicic acid. Anal Sci 5: 245–252
Udagawa N, Takahashi N, Akatsu N, Sasaki T, Yamaguchi A, Kodama H, Martin JT, Suda T (1989) The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology 125:1805–1813
Takahashi N, Yamana H, Yoshiki S, Roodman GD, Mundy GR, Jones SJ, Boyde A, Suda T (1988) Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology 122:1373–1382
Kodama Y, Takeuchi Y, Suzawa M, Fukumoto S, Murayama H, Yamato H, Fujita T, Kurowawa T, Matdumoto T (1998) Reduced expression of interleukin-11 in bone marrow stromal cells of senescence-accelerated mouse (SAMP6): relationship to osteopenia with enhanced adipogenesis. J Bone Miner Res 13:1370–1376
Sakiyama H, Masuda R, Inoue N, Yamamoto K, Kuriiwa K, Nakagawa K, Yoshida K (2001) Establishment and characterization of macrophage-like cell lines expressing osteoclast-specific markers. J Bone Miner Metab 19:220–227
Fukuyama S, Tashjian AH (1990) Stimulation by parathyroid hormone of 45Ca2+ uptake in osteoblast-like cells. Endocrinology 126:1941–1949
Podenphant J, Larsen N-E, Christiansen C (1984) An easy and reliable method for determination of urinary hydroxyproline. Clin Chim Acta 142:145–148
Nakamura T, Kurokawa T, Orimo H (1989) Increased mechanical strength of the vitamin D-replete rat femur by the treatment with a large dose of 24R, 25(OH)2D3. Bone (NY) 10:117–123
Taussky HH, Shorr E (1953) A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem 202:675–685
Woessner, JF Jr (1961) The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93:440–447
Hosokawa M, Abe T, Higuchi K, Shimakawa K, Omori Y, Matsushita T, Kogishi K, Deguchi E, Kishimoto Y, Yasuoka K, Takeda T (1997) Management and design of the maintenance of SAM mouse strains: an animal model for accelerated senescence and age-associated disorders. Exp Gerontol 32:111–116
Takami M, Takahashi N, Udagawa N, Miyaura C, Suda K, Woo J-T, Martin JT, Nagai K, Suda T (2000) Intracellular calcium and protein kinase C mediate expression of receptor activator of nuclear factor-κB ligand and osteoprotegerin in osteoblasts. Endocrinology 141:4711–4719
Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, Toyama Y, Taguchi T, Tanaka J (2005) The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 26:4847–4855
Takami M, Woo J-T, Takahashi N, Suda T, Nagai K (1997) Ca2+-ATPase inhibitors and Ca2+-ionophore induce osteoclast-like cell formation in the cocultures of mouse bone marrow cells and calvarial cells. Biochem Biophys Res Commun 237:111–115
Birchall JD (1992) The interrelationship between silicon and aluminium in the biological effects of aluminium. In: Chadwick DJ, Whelan J (eds) Aluminum in Biology and Medicine, Ciba Foundation Symposium 169. Wiley, Chichester, pp 50–68
Taylor PD, Jugdaohsingh R, Powell JJ (1997) Soluble silica with high affinity for aluminium under physiological and natural conditions. J Am Chem Soc 119:8852–8856
Yamaguchi A, Komori T, Suda T (2000) Regulation of osteoblast differentiation mediated by bone morphogenic proteins, hedgehogs and Cbfa 1. Endocr Rev 21:393–411
Gori F, Thomas T, Hicok KC, Spelsberg TC, Riggs BL (1999) Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment and inhibits late adipocyte maturation. J Bone Miner Res 14:1522–1535
Lee KS, Kim H-J, Li O-L, Chi X-Z, Ueta C, Komori T, Wozney JM, Kim E-G Choi J-Y, Ryoo H-M, Bae S-C (2000) Runx2 is a common target of transforming growth factor β1 and bone morphogenic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol 20: 8783–8792
Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi R (2006) BMP2 signaling is required for Runx2-dependent induction of the osteoblast phenotype. J Bone Miner Res 21:637–646
Ducy P, Karsenty G (1995) Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol 15:1858–1869
Harada H, Tagashira S, Fujiwara M, Ogawa S, Katsumata T, Yamaguchi A, Komori T, Nakatsuka M (1999) Cbfa 1 isoforms exert functional differences in osteoblast differentiation. J Biol Chem 74:6972–6978
Kern B, Shen J, Starbuck M, Karsenty G (2001) Cbfa 1 contributes to the osteoblast-specific expression of Type I collagen genes. J Biol Chem 276:7101–7107
Ducy P, Zang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Ocbfa 1: A transcriptional activator of osteoblast differentiation. Cell 89:747–754
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao Y-H, Inada M, Sato M, Okamoto R, Kitamura Y, Sato M, Okamoto R (1997) Targeted disruption of Cbfa 1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997;89:755–764
Xiao ZS, Thomas TK, Hinson TK, Quarles LD (1998) Genomic structure and isoforms expression of mouse, rat and human Cbfa 1/Osf2 transcription factor. Gene (Amst) 214:187–197
Du X, Williams DA (1977) Interleukin-11: review of molecular, cell biology, and clinical use. Blood 11:3897–3908
Keller DC, Du XX, Srour EF, Hoffman R, Williams DA (1993) Interleukin-11 inhibits adipogenesis and stimulates myelpoiesis in long term marrow cultures. Blood 82:1428–1435
Ohsumi J, Miyadai K, Kawashima I, Ishikawa-Ohsumi H, Sakakibara S, Mita-Honjo K, Takiguchi Y (1991) Adipogenesis inhibitory factor. A novel inhibitory regulator of adipose conversion in bone marrow. FEBS Lett 288:13–16
Girasole G, Passeri G, Jilka RL, Manolagas SC (1994) Interleukin-11: a new cytokine critical for osteoclast development. J Clin Invest 93:1516–1524
Verhaeghe J, Van Herck E, Van Bree R, Bouillon R, Dequeker J, Keith JC Jr (1998) rHuIL-11 does not modify biochemical parameters of bone remodeling and bone mineral density in adult ovariectomized rats. J Interferon Cytokine Res 18:49–53
Takeuchi Y, Watanabe S, Ishii G, Takeda S, Nakayama K, Fukumoto S, Kaneta Y, Inoue D, Matsumoto T, Harigaya Y, Fujita T (2002) Interleukin-11 as a stimulatory factor for bone formation prevents bone loss with advancing age in mice. J Biol Chem 277:49011–49018
Suga K, Saitoh M, Fukushima S, Takahashi K, Nara H, Yasuda S, Miyata K (2001) Interleukin-11 induces osteoblast differentiation and acts synergistically with bone morphogenic protein-2 in C3H10T1/2 cells. J Interferon Cytokine Res 21:659–707
Yasuda H, Shima N, Nakagawa N, Yamaguchi M, Kinosaki M, Goto M, Mochizuki S-I, Tsuda E, Morinaga T, Udagawa N, Takahashi N, Suda T, Higashio K (1999) A novel molecular mechanism modulating osteoclast differentiation and function. Bone (NY) 25:109–113
Yasuda H, Shima N, Nakagawa N, Mochizuki S-I, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K (1998) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139:1329–1337
Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141: 3478–3484
Horwood NJ, Elliott J, Martin TJ, Gillespie MT (1998) Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stomal cells. Endocrinology 139:4743–4746
Silver IA, Murrills RJ, Etherington DJ (1988) Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res 175:266–276
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Maehira, F., Iinuma, Y., Eguchi, Y. et al. Effects of soluble silicon compound and deep-sea water on biochemical and mechanical properties of bone and the related gene expression in mice. J Bone Miner Metab 26, 446–455 (2008). https://doi.org/10.1007/s00774-007-0845-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-007-0845-x