Abstract
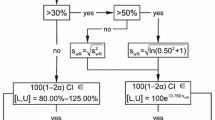
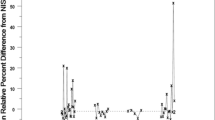
The possibility of using interlaboratory study repeatability and reproducibility estimates as the basis for measurement uncertainty estimates is discussed. It is argued that collaborative trial reproducibility is an appropriate basis for estimating uncertainty in routine testing provided certain conditions are met by the laboratory. The primary shortcomings relate to establishment of traceability and consequent estimation of bias associated with the method, and quantitatively establishing the relevance to the single laboratory. Approaches to resolving both difficulties are proposed, the former via full implementation of trueness determination suggested in ISO 5725 : 1994 or by independent checks on individual accuracy and precision, the latter via a reconciliation procedure. The paper also discusses other factors including sampling and sample pre-treatment, change in sample matrix, and the influence of level of analyte.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 28 October 1997 · Accepted: 17 November 1997
Rights and permissions
About this article
Cite this article
Ellison, S. ISO uncertainty and collaborative trial data. Accred Qual Assur 3, 95–100 (1998). https://doi.org/10.1007/s007690050197
Issue Date:
DOI: https://doi.org/10.1007/s007690050197