Abstract
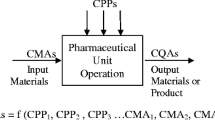
A process is well understood when all critical sources of variability are identified and explained, variability is managed by the process design and monitoring, and product quality attributes are accurately and reliably predicted over the design space. Quality by Design (QbD) is a systematic approach to product development and process control that begins with predefined objectives, emphasizes product and process understanding, and sets up process control based on sound science and quality risk management. The Food and Drug Administration (FDA) and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) have recently started promoting QbD in an attempt to curb rising development costs and regulatory barriers to innovation and creativity. QbD is partially based on the application of multivariate statistical methods and a statistical Design of Experiments strategy to the development of both analytical methods and pharmaceutical formulations. In this paper, we review the basics of QbD and their impact on the innovative, generic, and biosimilar pharmaceutical industry. In particular, we consider the challenge of mapping the control space in biotechnological processes and how advances in statistical methods can contribute to QbD.


Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- CQA:
-
Critical quality attribute
- DP:
-
Drug product
- FDA:
-
Food and Drug Administration
- ICH:
-
The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use
- QbD:
-
Quality by Design
- VoC:
-
Voice of the customer
References
Bates R, Kenett R, Steinberg D, Wynn H (2006) Qual Technol Quant Manage 3(2):161–177
Borman P, Nethercote P, Chatfield M, Thompson D, Truman K (2007) Pharm Technol. Available online at: http://pharmtech.findpharma.com/pharmtech/Peer-Reviewed+Research/The-Application-of-Quality-by-Design-to-Analytical/ArticleStandard/Article/detail/463580
Cook S, Patton KA, Bazemore LR (2007) BioPharm Int 20(12):28–37. Available online at: http://biopharminternational.findpharma.com/biopharm/Outsourcing/Quality-by-Design-in-the-CMO-Environment/ArticleStandard/Article/detail/478714
Food and Drug Administration (2006) Guidance for industry quality systems approach to pharmaceutical cGMP regulations. Available online at: http://www.fda.gov/CDER/guidance/7260fnl.htm
Fuchs C, Kenett R (1988) Statistician 37:401–411
Fuchs C, Kenett R (1998) J Qual Technol 19:122–131
Fuchs C, Kenett R (1998) Multivariate quality control: theory and applications. Marcel Dekker Inc., New York
Huang D, Allen T (2005) J R Stat Soc C 54(2):443–463
ICH (2006) The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Quality Guideline Q8 Pharmaceutical Development. Available online at: http://www.fda.gov/CbER/gdlns/ichq8pharm.htm
Kenett R, Stark Y (2008) Drug development strategy: the interface between QbD and statistical methods. In: Proceedings of the Global Pharmaceutical Conference on Practical Application of QbD in Accelerated Development, Frankfurt, Germany, April 2008. University of Wisconsin, School of Pharmacy, Madison
Kenett R, Steinberg D (2006) Quality progress, August, pp 61–65
Kenett R, Zacks S (1998) Modern industrial statistics: design and control of quality and reliability. Duxbury Press, San Francisco. Spanish edn 2000, 2nd paperback edn 2002, Chinese edn 2004
Kennedy M, O’Hagan A (2000) Biometrika 87:1–13
Myers R, Montgomery D, Vining G, Borror C, Kowalski S (2004) J Qual Technol 36(1):53–77
Nasr M (2007) Quality by Design (QbD)—a modern system approach to pharmaceutical development and manufacturing: FDA perspective. FDA Quality Initiatives Workshop, Maryland
Nethercote P (2008) Implications of QbD approach on analytical method development, validation, transfer and change control. In: Proceedings of the Global Pharmaceutical Conference on Practical Application of QbD in Accelerated Development, Frankfurt, Germany, April 2008. University of Wisconsin, School of Pharmacy, Madison
Niazi K (2006) Handbook of biogeneric therapeutic proteins regulatory, manufacturing, testing, and patent issues. CRC Press, Boca Raton, FL
Orchard T (2006) BioPharm 19:34–46
Reese C, Wilson A, Hamada M, Martz H, Ryan K (2004) Technometrics 46(2):153–164
Romero R, Gasquez D, Sanshez M, Rodriguez L, Bagur M (2002) LC GC North Am 20:72–80
Ruggeri F, Kenett R, Faltin F (eds) (2007) Wiley encyclopedia of statistics in quality and reliability. Wiley, London
Sacks J, Welch W, Mitchell T, Wynn H (1989) Stat Sci 4:409–435
Santner TJ, Williams BJ, Notz WI (2003) The design and analysis of computer experiments. Springer, New York
Schweitzer M (2008) Quality by design for analytical methods—an industry perspective (PhRMA). In: Proceedings of the Global Pharmaceutical Conference on Practical Application of QbD in Accelerated Development, Frankfurt, Germany, April 2008. University of Wisconsin, School of Pharmacy, Madison
Yu L (2008) Pharm Res 25(4):781–791
Author information
Authors and Affiliations
Corresponding author
Appendix: software products for simulations and QbD statistical analysis
Appendix: software products for simulations and QbD statistical analysis
Simulation experiments:
-
VisiMix: http://www.visimix.com/
-
DynoChem: http://www.scale-up.com/index.html
Statistical design of experiments:
-
JMP: http://www.jmp.com/
-
MINITAB: http://www.minitab.com/
-
Design-Expert: http://www.statease.com/dx71descr.html
-
Empower 2 Method Validation Manager: http://www.waters.com/waters/nav.htm?locale=en_US&cid=534328
Multivariate methods:
-
Clementine: http://www.spss.com/clementine/capabilities.htm
-
GeNie: http://genie.sis.pitt.edu
-
TITOSIM: http://www.titosim.com/
Rights and permissions
About this article
Cite this article
Kenett, R.S., Kenett, D.A. Quality by Design applications in biosimilar pharmaceutical products. Accred Qual Assur 13, 681–690 (2008). https://doi.org/10.1007/s00769-008-0459-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-008-0459-6