Abstract
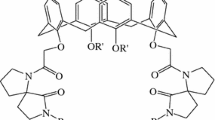
The task of this work was to investigate the extraction capacity of various calixarenes for free and esterified amino acids from aqueous acid phases. Furthermore, this method was applied to aqueous extracts of Helleborus purpurascens. Generally, it is known that calixarenes can be used as extractants for ammonium compounds due to π-cation and lone pair cation interactions. As first, tert-Butyl-calix[6]arene and derivatives thereof were used. They had already proven their worth in previous investigations. In addition, tert-Butyl-hexahomooxa-calix[3]arene was used also, which can also enter into lone pair cation interactions. In addition to these well-known calixarenes, new calixarenes were produced and tested. Based on the tert-Butyl-hexahomooxa-calix[3]arene, a phosphor(III)bridged derivative was prepared, combining the three aromatic hydroxyl groups to a phosphite. As a seldom-described class of calixarenes, tert-Butyl-hexahomoaza-calix[3]arene derivatives were used. The nitrogen analogues of tert-Butyl-hexahomooxa-calix[3]arene could be produced as N-benzyl derivatives. The structure of the esterified carboxymethylated derivative of N,N′,N″-Tribenzyl-tert-Butyl-hexahomoaza-calix[3]arene could be verified by X-ray structure analysis. It crystallized as a partial cone. The extraction capacity of the described calixarenes was investigated for amino acids from aqueous acidic solutions into an organic phase. For the testing were chosen asparagine, aspartic acid, tyrosine, tryptophane, phenylalanine and pipecolinic acid and their methyl esters. The amino acids and their methyl esters were dissolved in water at different pH values. The calixarenes were dissolved in dichloromethane (DCM) or chloroform. After this preparation, the aqueous acidic amino acid solutions were mixed with the solutions and shaken intensively. In addition, blank values were determined by extracting the aqueous stock solutions of the amino acids and their methyl esters with pure solvents. To determine the extraction rate, the phases were separated and each analysed using GC-FID, partially GC–MS(EI). The evaluation is performed in two ways. On the one hand the depletion in the aqueous phase and on the other hand the content in the organic phase was determined.










Similar content being viewed by others
Notes
The term “hydroalcoholic” is not precisely but commonly used. Therefore, the term is also used by the authors for the extract which was generated with a mixture of water and ethanol.
A congruence of retention time and fragmentation pattern between an unknown substance and an authentic material proves the identity of an unknown substance.
References
Araki K, Inada K, Otsuka H, Shinkai S (1993a) Conformational isomerism in and binding properties to alkali-metals and an ammonium salt of O-alkylated homooxacalix[3]arenes. Tetrahedron 49:9465–9478
Araki K, Hashimoto N, Otsuka H, Shinkai S (1993b) Synthesis and ion selectivity of conformers derived from hexahomotrioxacalix[3]arene. J Org Chem 58:5958–5963
Araki K, Inada K, Shinkai S (1996) Chiral recognition of a-amino acid derivatives with a homooxacalix[3]arene: construction of a pseudo-C,-symmetrical compound from a C,-symmetrical macrocycle. Angew Chem Int Ed Engl 35:72–74
Arnaud-Neu F, Browne JK, Byrne D, Marrs DJ, McKervey MA, Hagan PO, Schwing-Weill MJ, Walker A (1999) Extraction and complexation of alkali, alkaline Earth, and F-element cations by calixaryl phosphine oxides. Chem Eur J 5:175–176
Arnott GE (2018) Inherently chiral calixarenes: synthesis and applications. Chem Eur J 24:1744–1754
Capasso S, Mazzarella L, Sica F, Zagari F (1989) Chiroptical properties of aminosuccinyl peptides. Int J Pept Protein Res 33:124–132
Chirakul P, Hampton PD, Bencze Z (2000) A convergent synthesis of hexahomotriazacalix[3]arene macrocycles. J Org Chem 65:8297–8300
Dieleman CB, Matt D, Neda I, Schmutzler R, Harriman A, Yaftian R (1999) Hexahomotrioxacalix[3]arene: a scaffold for a C3-symmetric phosphine ligand that traps a hydro-rhodium fragment inside a molecular funnel. Chem Commun 18:1911–1912
Dunn MS, Ross FJ (1938) Quantitative investigations of amino acids and peptides: IV. The solubility of amino acids in water - ethyl alcohol mixtures. J Biol Chem 125:309–332
Durmaz M, Halay E, Bozkurt S (2018) Recent applications of chiral calixarenes in asymmetric catalysis. Beilstein J Org Chem 14:1389–1412
Franz MH, Birzoi R, Maftei CV, Maftei E, Kelter G, Fiebig HH, Neda I (2018) Studies on the constituents of Helleborus purpurascens: analysis and biological activity of the aqueous and organic extracts. Amino Acids 50:163–188
Germann UA, Shlyakhter D, Mason VS, Zelle RE, Duffy JP, Galullo V et al (1997) Cellular and biochemical characterization of VX-710 as a chemosensitizer: reversal of P-glycoprotein-mediated multidrug resistance in vitro. Anticancer Drugs 8:125–140
Gopalakrishnan R, Kozany C, Gaali S, Kress C, Hoogeland B, Bracher A, Hausch F (2012) Evaluation of synthetic FK506 analogues as ligands for the FK506-binding proteins 51 and 52. J Med Chem 55:4114–4122
Kadyrov A, Neda I, Kaukorat T, Soodenburg R, Fischer A, Jones PG, Schmutzler R (1996) New phospholene and phosphepine derivatives irom λ3-phosphorus compounds and hexafluoroacetone or periluorinated α-diketones. Chem Ber 129:725–732
Kawaguchi M, Ikeda A, Shinkai S, Neda I (2000) Electrochemical studies of calixarene-[60]fullerene inclusion processes. J Incl Phenom Mol Recognit 37:253–258
Kerek F (2000) The structure of the digital is like and natriuretic factors identified as macrocyclic derivatives of the inorganic carbon suboxide. Hypertens Res 23:S33–S38
Kerek F, Szegli G, Cremer L, Lupu AR, Durbaca S, Calugaru A, Herold A, Radu DL (2008) The novel arthritis-drug substance MCS-18 attenuates the antibody production in vivo. Acta Microbiol Immunol Hung 55:15–31
Khan IU, Takemura H, Suenaga M, Shinmyozu T, Inazu T (1993) Azacalixarenes: new macrocycles with dimethyleneaza-bridged calix[4]arene systems. J Org Chem 58:3158–3161
Kunze C, Selent D, Neda I, Schmuzler R, Spannenberg A, Börner A (2001) Synthesis of new calix[4]arene-based phosphorus ligands and their application in the Rh(I) catalyzed hydroformylation of 1-Octene. Heteroat Chem 12:577–585
Li J, Sha Y (2008) A convenient synthesis of amino acid methyl esters. Molecules 13:1111–1119
Macarie L, Plesu N, Iliescu S, Ilia G (2017) Synthesis of organophosphorus compounds using ionic liquids. Rev Chem Eng 34:727–740
Maftei CV, Fodor E, Jones PG, Franz MH, Davidescu CM, Neda I (2015) Asymmetric calixarene derivatives as potential hosts in chiral recognition processes. Pure Appl Chem 87:415–439
Maftei CV, Fodor E, Jones PG, Freytag M, Franz MH, Kelter G, Fiebig HH, Tamm M, Neda I (2016a) Novel 1,2,4-oxadiazoles and trifluoromethylpyridines related to natural products: synthesis, structural analysis and investigation of their antitumor activity. Tetrahedron 72:1185–1199
Maftei E, Maftei CV, Jones PG, Freytag M, Franz MH, Kelter G, Fiebig HH, Tamm M, Neda I (2016b) Trifluoromethylpyridine-substituted N-heterocyclic carbenes related to natural products: synthesis, structure and potential antitumor activity of some corresponding gold(I), rhodium(I) and iridium(I) complexes. Helv Chim Acta 99:469–481
Morohashi N, Hattori T (2018) Selective guest inclusion by crystals of calixarenes: potential for application as separation materials. J Incl Phenom Macrocycl Chem 90:261–277
Muhr P, Kerek F, Dreveny D, Likussar W, Schubert-Zsilavecz M (1995) The structure of hellebrin. Liebigs Ann 1995:443–444
Mutihac L, Lee JH, Kim JS, Vicens J (2011) Recognition of amino acids by functionalized calixarenes. Chem Soc Rev 40:2777–2796
Návarová H, Bernsdorff F, Döring AC, Zeier J (2012) Pipecolic acid, an endogenous mediator of defence amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24:5123–5141
Neacsu C, Ciobanu C, Barbu I, Toader O, Szegli G, Kerek F, Babes A (2010) Substance MCS-18 isolated from Helleborus purpurascens is a potent antagonist of the capsaicin receptor, TRPV1, in rat cultured sensory neurons. Physiol Res 59:289–298
Neda I, Farkens M, Fischer A, Jones PG, Schmutzler R (1993) Chemistry of the l,3,5-triaza-2-phosphinane-4,6-diones, part V synthesis of phosphoryl(III) (λ4P) and thiophosphoryl(III) (λ4P) derivatives of 1,3,5-triaza-2-phosphinane-4,6-diones. Reactions with ketones. Z Naturforsch 48b:860–866
Neda I, Melnicky C, Vollbrecht A, Schmutzler R (1996) An unusual N-alkylation reaction during the oxidative addition of hexafluoroacetone and tetrachloro-o-benzoquinone to P-bis(2-chloroethyl)amino-substituted λ3P-compounds. Synthesis 1996:473–474
Neda I, Vollbrecht A, Grunenberg J, Schmutzler R (1998) Functionalization of the periphery of calix[4]resorcinarenes with P(III)-containing substituents via hydroxy, trimethylsiloxy, and ethoxy-tethered trimethylsiloxy intermediates. Heteroat Chem 9:553–558
Neda I, Sakhaii P, Waßmann A, Niemeyer U, Günther E, Engel J (1999) A practical synthesis of benzyl α- and allyl β-D-glucopyranosides regioselectively substituted with (CH2)3OH groups Stereocontrolled β-galactosidation by cation π-interaction. Synthesis 1999:1625–1632
Neda I, Kaukorat T, Schmutzler R, Niemeyer U, Kutscher B, Pohl J, Engel J (2000) Benzodiaza-, benzoxaza-, and benzodioxaphosphorinones—formation, reactivity, structure, and biological activity. Phosphorus Sulfur Silicon Relat Elem 162:81–218
Nernst W (1891) Verteilung eines Stoffes zwischen zwei Lösungsmitteln und zwischen Lösungsmittel und Dampfraum. Z Phys Chem 8:110–139
Nozaki Y, Tanford C (1971) The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions establishment of a hydrophobicity scale. J Biol Chem 246:2211–2217
Oshima T (2014) Protein extraction by the recognition of lysine residues using macrocyclic molecules. Ion Exch Solvent Extr 21:129–158
Oshima T, Goto M, Furusaki S (2002) Extraction behavior of amino acids by calix[6]arene carboxylic acid derivatives. J Incl Phenom Macrocycl Chem 43:77–86
Otsuka H (2005) Purification by solvent extraction using partition coefficient. Natural Products Isolation. Methods in Biotech 20:269–273
Schmutz J (1947) Glycosides and aglycones. XXII. Hellebrin. Pharm Acta Hel 22:373–380
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A64:112–122
Siedentop T, Neda I, Thönnessen H, Jones PG, Schmutzler R (1999) Synthesis and X-ray structure analysis of the first tert-butylcalix[4]arene salts with a phosphonium cation. Z Naturforschung 54b:761–766
Takemura H, Yoshimura K, Khan IU, Shinmyozu T, Inazu T (1992) The first synthesis and properties of hexahomotriazacalix[3]arene. Tetrahedron Lett 33:5775–5778
Vollbrecht A, Neda I, Thönnessen H, Jones PG, Harris RK, Crowe LA, Schmutzler R (1997) Synthesis, structure, and reactivity of tetrakis(O, O-phosphorus)-bridged calix[4]resorcinols and their derivatives. Chem Berichte 130:1715–1720
Zeier J (2013) New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ 36:2085–2103
Acknowledgements
The authors want to thank Ing. Geta Serbanescu and Ing. Draga Todorov (both S.C. Exhelios S.R.L.) for fruitful discussions and for the support of this work with rhizome and roots of H. purpurascens and an already prepared hydroalcoholic extract thereof. The authors want to thank also Dr. Macarie A. Corina and Dr. Ionel Balcu, (both: Institutul National de Cercetare Dezvoltare pentru Electrochimie si Materie Condensata, Timisoara, Romania) for their contributions for this publication and also Dr. Matthias Freytag (Institut für Anorganische und Analytische Chemie, Technische Universität Braunschweig, Braunschweig, Germany); the authors want to thank for the measuring of the x-ray structure. In addition, this work was supported by the Romanian National Authority for Scientific Research through the exploratory research program: “DEI-PCE-PROJECT NR. 341-/05.10.2011-Immunomodulatory Fluoroglycopeptide Molecular Architectures and PROJECT NR. 18360301, Contract: 42N/2018”.
Author information
Authors and Affiliations
Contributions
IN, MI and MHF contributed to the study conception and design. EM synthesised the Homoaza-calix[3]arene; IN and CVM synthesised the phosphorous-bridged Homooxa-calix[3]arene, MHF synthesised the esters of the amino acids; excepted the extractions and performed the GC analysis. The first draft of the manuscript was written by MHF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Handling Editor: V. Soloshonok.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Franz, M.H., Iorga, M., Maftei, C.V. et al. Studies on the constituents of Helleborus purpurascens: use of derivatives from calix[6]arene, homooxacalix[3]arene and homoazacalix[3]arene as extractant agents for amino acids from the aqueous extract. Amino Acids 52, 55–72 (2020). https://doi.org/10.1007/s00726-019-02809-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-019-02809-z