Abstract
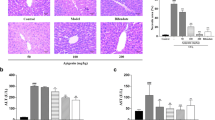
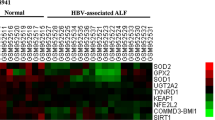
Hepatocyte apoptosis plays a key role in the pathogenesis of immune-mediated hepatitis. However, the detailed mechanisms of apoptosis signaling are still unclear and effective therapeutic drugs for hepatitis have been explored. Here, we show that tryptophan (Trp) suppressed IFN-γ-mediated hepatic apoptosis in vitro. Trp inhibited the downstream apoptotic events of mitochondria disruption, such as cell death and caspase-3 activation, while it did not influence upstream signaling including STAT1 activation and IRF1 expression. Trp suppressed reactive oxygen species (ROS) generation at the mitochondria. IFN-γ induced ROS in mitochondria by inhibiting complex I and III, but not II. This ROS generation by IFN-γ required de novo protein synthesis. Trp showed relatively weak direct scavenging activity but antagonized IFN-γ against the suppression of complex I. In addition, Trp increased the expression of the Nrf2-dependent antioxidant genes NQO1, HO-1 and GCS in hepatocytes both in vitro and in vivo. Finally, the administration of Trp in an acetaminophen-induced ROS-dependent hepatitis model suppressed the liver injury in vivo. Thus, Trp protects hepatocytes from ROS-dependent cell injury via multiple pathways. This study suggests Trp as a therapeutic antioxidant drug for hepatitis and a regulator for Nrf2-dependent genes.








Similar content being viewed by others

References
Atia A, Alrawaiq N, Abdulllah A (2014) A review of NAD(P)H: quinone oxidoreductase 1 (NQO1); a multifunctional antioxidant enzyme. J Appl Pharm Sci 4:118–122
Baird L, Dinkova-Kostova AT (2011) The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol 85:241–272
Bartolini D, Commodi J, Piroddi M, Incipini L, Sancineto L, Santi C, Galli F (2015) Glutathione S-transferase pi expression regulates the Nrf2-dependent response to hormetic diselenides. Free Radic Biol Med 88:466–480. doi:10.1016/j.freeradbiomed.2015.06.039
Bender DA (1982) Biochemistry of tryptophan in health and disease. Mol Aspects Med 6:101–197
Benson AM, Hunkeler MJ, Talalay P (1980) Increase of NAD(P)H: quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci USA 77:5216–5220
Bessede A et al (2014) Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511:184–190
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol 28:25–30
Car BD et al (1994) Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med 179:1437–1444
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria. Central role of complex III. J Biol Chem 278:36027–36031
Crunkhorn S (2012) Deal watch: Abbott boosts investment in NRF2 activators for reducing oxidative stress. Nat Rev Drug Discov 11:96
Decker T, Lohmann-Matthes M-L (1988) A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods 15:61–69
Ehlers S, Mielke ME, Blankenstein T, Hahn H (1992) Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. J Immunol 149:3016–3022
Ferri KF, Kroemer G (2001) Organelle-specific initiation of cell death pathways. Nat Cell Biol 3:E255–E263
Genestet C, Gouellec AL, Chaker H, Polack B, Guery B, Toussaint B, Stasia MJ (2014) Scavenging of reactive oxygen species by tryptophan metabolites helps Pseudomonas aeruginosa escape neutrophil killing. Free Radic Biol Med 73:400–410
Grewal P, Mallaney M, Lau K, Sreedhara A (2014) Screening methods to identify indole derivatives that protect against reactive oxygen species induced tryptophan oxidation in proteins. Mol Pharm 11:1259–1272
Grohmann U, Bronte V (2010) Control of immune response by amino acid metabolism. Immunol Rev 236:243–264
Holmstroem KM et al (2013) Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open 2:761–770
Ihle JN, Kerr IM (1995) Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet 11:69–74
Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Thierfelder WE, Kreider B, Silvennoinen O (1994) Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci 19:222–227
Inai Y, Yabuki M, Kanno T, Akiyama J, Yasuda T, Utsumi K (1997) Valinomycin induces apoptosis of ascites hepatoma cells (AH-130) in relation to mitochondrial membrane potential. Cell Strucut Funct 22:555–563
Kanki K, Kawamura T, Watanabe Y (2009) Control of ER stress by a chemical chaperone counteracts apoptotic signals in IFN-γ-treated murine hepatocytes. Apoptosis 14:309–319
Kano A, Haruyama T, Akaike T, Watanabe Y (1999) IRF-1 is an essential mediator in IFN-γ-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem Biophys Res Commun 257:672–677
Kanzaki H, Shinohara F, Kajiya M, Kodama T (2013) The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J Biol Chem 288:23009–23020
Korzeniewski C, Callewaert DM (1983) An enzyme-release assay for natural cytotoxicity. J Immunol Methods 64:313–320
Kovac S, Angelova PR, Holmstroem KM, Zhang Y, Dinkova-Kostova AT, Abramov AY (2015) Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta 1850:794–801
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6:513–519
Lee KK, Imaizumi N, Chamberland SR, Alder NN, Boelsterli UA (2015) Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology 61:326–336
Lemasters JJ et al (1998) The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366:177–196
Li X, Fang P, Mai J, Choi ET, Wang H, Yang X (2013) Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol 6:19
Luo C, Long J, Liu J (2008) An improved spectrophotometric method for a more specific and accurate assay of mitochondrial complex III activity. Clin Chim Acta 395:38–41
Mathers J, Fraser JA, McMahon M, Saunders RDC, Hayes JD, McLellan LI (2004) Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp 71:157–176
McClain CJ, Cohen DA (1989) Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology 9:349–351
Mizuhara H et al (1996) Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mehanisms of interleukin-6 for the manifestations of hepatitis. Hepatology 23:1608–1615
Morita M, Watanabe Y, Akaike T (1994) Inflammatory cytokines up-regulate intercellular adhesion molecule-1 expression on primary cultured mouse hepatocytes and T-lymphocyte adhesion. Hepatology 19:426–431
Morita M, Watanabe Y, Akaike T (1995) Protective effect of hepatocyte growth factor on interferon-gamma-induced cytotoxicity in mouse hepatocytes. Hepatology 21:1585–1593
Pilotte L et al (2012) Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA 109:2497–2502
Schneider-Helmert D, Spinweber CL (1986) Evaluation of l-tryptophan for treatment of insomnia: a review. Pshycopharmacology 89:1–7
Shin SM, Yang JH, Ki SH (2013) Role of the Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev 2013:763257. doi:10.1155/2013/763257
Tagawa Y, Sekikawa K, Iwakura Y (1997) Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice. Role for IFN-γ in activating apoptosis of hepatocytes. J Immunol 159:1418–1428
Toyonaga T, Hino O, Sugai S, Wakasugi S, Abe K, Shichiri M, Yamamura K (1994) Chronic active hepatitis in transgenic mice expressing interferon-γ in the liver. Proc Natl Acad Sci USA 91:614–618
Watanabe Y, Suzuki O, Haruyama T, Akaike T (2003) Interferon-g induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J Cell Biochem 89:244–253
Yates MS et al (2009) Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis 30:1024–1031
Acknowledgments
Financial support for this work was provided by Musashino University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Additional information
Handling Editor: F. Galli.
Rights and permissions
About this article
Cite this article
Kimura, T., Watanabe, Y. Tryptophan protects hepatocytes against reactive oxygen species-dependent cell death via multiple pathways including Nrf2-dependent gene induction. Amino Acids 48, 1263–1274 (2016). https://doi.org/10.1007/s00726-016-2175-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2175-6