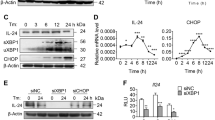
Abstract
Apoptosis of hepatocytes plays a key role in the pathogenesis of immune-mediated hepatitis. However, the detailed mechanisms of apoptotic signaling remain unclear. In this study, we investigated the involvement of ER stress in a model of IFN-γ-induced apoptosis of hepatocytes in vitro, using a chemical chaperone reagent, glycerol. IFN-γ-induced apoptotic events (mitochondrial release of cytochrome c, enzymatic activation of caspase-3 and -9) were markedly inhibited by glycerol. Glycerol induced partial inhibition of cytotoxicity indicated by lactate dehydrogenase release from the cytosol but had no inhibitory effect on the induction of IRF-1 gene expression and reactive oxygen species, required for hepatocyte apoptosis by IFN-γ. Induction of caspase-4 and -12 gene expression, positively correlated with ER stress, was attenuated by glycerol. Gene analysis revealed that induction of ER stress-related genes, C/EBP homologue protein (CHOP/GADD153) and TRB3, was suppressed completely by glycerol treatment. These results suggest that ER stress plays a crucial role in mediating apoptosis of hepatocytes induced by IFN-γ, and a chemical chaperone is an effective inhibitor of the ER stress.







Similar content being viewed by others
Abbreviations
- AG:
-
Aminoguanidine
- CHOP:
-
C/EBP homologue protein
- ER:
-
Endoplasmic reticulum
- IFN-γ:
-
Interferon gamma
- LDH:
-
Lactate dehydrogenase
- 4-PBA:
-
Sodium 4-phenylbutyrate
- PDTC:
-
Pyrrolidinedithiocarbamate
- ROS:
-
Reactive oxygen species
- SERCA:
-
Sarcoplasmic/endoplasmic Ca2+-ATPase
- TG:
-
Thapsigargin
- UPR:
-
Unfold protein response
References
Morita M, Watanabe Y, Akaike T (1995) Protective effect of hepatocyte growth factor on interferon-gamma-induced cytotoxicity in mouse hepatocytes. Hepatology 21:1585–1593
McClain CJ, Cohen DA (1989) Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology 9:349–351. doi:10.1002/hep.1840090302
Ehlers S, Mielke ME, Blankenstein T et al (1992) Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. J Immunol 149:3016–3022
Mizuhara H, Uno M, Seki N et al (1996) Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology 23:1608–1615
Car BD, Eng VM, Schnyder B et al (1994) Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med 179:1437–1444. doi:10.1084/jem.179.5.1437
Tagawa Y, Sekikawa K, Iwakura Y (1997) Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice. Role for IFN-γ in activating apoptosis of hepatocytes. J Immunol 159:1418–1428
Toyonaga T, Hino O, Sugai S et al (1994) Chronic active hepatitis in transgenic mice expressing interferon-γ in the liver. Proc Natl Acad Sci USA 91:614–618. doi:10.1073/pnas.91.2.614
Kano A, Watanabe Y, Takeda N et al (1997) Analysis of IFN-γ-induced cell cycle arrest and cell death in hepatocytes. J Biochem 121:677–683
Ihle JN, Witthuhn BA, Quelle FW et al (1994) Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci 19:222–227. doi:10.1016/0968-0004(94)90026-4
Ihle JN, Kerr IM (1995) Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet 11:69–74. doi:10.1016/S0168-9525(00)89000-9
Kano A, Haruyama T, Akaike T et al (1999) IRF-1 is an essential mediator in IFN-γ-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem Biophys Res Commun 257:672–677. doi:10.1006/bbrc.1999.0276
Watanabe Y, Suzuki O, Haruyama T et al (2003) Interferon-γ induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J Cell Biochem 89:244–253. doi:10.1002/jcb.10501
Sitia R, Braakman I (2003) Quality control in the endoplasmic reticulum protein factory. Nature 426:891–894. doi:10.1038/nature02262
Szegezdi E, Logue SE, Gorman AM et al (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7:880–885. doi:10.1038/sj.embor.7400779
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110:1389–1398
Gekko K, Timasheff SN (1981) Mechanism of protein stabilization by glycerol: preferential hydration in glycerol–water mixtures. Biochemistry 20:4667–4676. doi:10.1021/bi00519a023
Meng FG, Hong YK, He HW et al (2004) Osmophobic effect of glycerol on irreversible thermal denaturation of rabbit creatine kinase. Biophys J 87:2247–2254. doi:10.1529/biophysj.104.044784
Shearer AG, Hampton RY (2004) Structural control of endoplasmic reticulum-associated degradation: effect of chemical chaperones on 3-hydroxy-3-methylglutaryl-CoA reductase. J Biol Chem 279:188–196. doi:10.1074/jbc.M307734200
Tamarappoo BK, Verkman AS (1998) Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest 101:2257–2267. doi:10.1172/JCI2303
Morita M, Watanabe Y, Akaike T (1994) Inflammatory cytokines up-regulate intercellular adhesion molecule-1 expression on primary cultured mouse hepatocytes and T-lymphocyte adhesion. Hepatology 19:426–431
Decker T, Lohmann-Matthes M-L (1988) A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods 15:61–69. doi:10.1016/0022-1759(88)90310-9
Korzeniewski C, Callewaert DM (1983) An enzyme-release assay for natural cytotoxicity. J Immunol Methods 64:313–320. doi:10.1016/0022-1759(83)90438-6
Ohoka N, Yoshii S, Hattori T et al (2005) TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24:1243–1255. doi:10.1038/sj.emboj.7600596
Corbett JA, Tilton RG, Chang K et al (1992) Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes 41:552–556. doi:10.2337/diabetes.41.4.552
Endo M, Mori M, Akira S et al (2006) C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol 176:6245–6253
Jiang S, Xie Q, Zhou H et al (2008) Ribozyme-mediated inhibition of caspase-12 activity reduces apoptosis induced by endoplasmic reticulum stress in primary mouse hepatocytes. Int J Mol Med 22:717–724
Rao RV, Hermel E, Castro-Obregon S et al (2001) Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem 276:33869–33874. doi:10.1074/jbc.M102225200
Cardozo AK, Ortis F, Storling J et al (2005) Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic b-cells. Diabetes 54:452–461. doi:10.2337/diabetes.54.2.452
Burrows JAJ, Willis LK, Perlmutter DH (2000) Chemical chaperones mediate increased secretion of mutant α1-antitrypsin (α1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in α1-AT deficiency. Proc Natl Acad Sci USA 97:1796–1801. doi:10.1073/pnas.97.4.1796
Qi X, Hosoi T, Okuma Y et al (2004) Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol 66:899–908. doi:10.1124/mol.104.001339
Tatzelt J, Prusiner SB, Welch WJ (1996) Chemical chaperones interfere with the formation of scrapie prion protein. EMBO J 15:6363–6373
Boyce M, Bryant KF, Jousse C et al (2005) A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307:935–939. doi:10.1126/science.1101902
Yamaguchi H, Wang HG (2004) CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279:45495–45502. doi:10.1074/jbc.M406933200
Zinszner H, Kuroda M, Wang X et al (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12:982–995. doi:10.1101/gad.12.7.982
Corcoran CA, Luo X, He Q et al (2005) Genotoxic and endoplasmic reticulum stresses differentially regulate TRB3 expression. Cancer Biol Ther 4:1063–1067
Kim S, Zhang Z, Hitomi E et al (2006) Endoplasmic reticulum stress-induced caspase-4 activation mediates apoptosis and neurodegeneration in INCL. Hum Mol Genet 15:1826–1834. doi:10.1093/hmg/ddl105
Hitomi J, Katayama T, Eguchi Y et al (2004) Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J Cell Biol 165:347–356. doi:10.1083/jcb.200310015
Gotoh T, Terada K, Oyadomari S et al (2004) Hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ 11:390–402. doi:10.1038/sj.cdd.4401369
McCullough KD, Martindale JL, Klotz LO et al (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259. doi:10.1128/MCB.21.4.1249-1259.2001
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529. doi:10.1038/nrm2199
Bajt ML, Knight TR, Lemasters JJ et al (2004) Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci 80:343–349. doi:10.1093/toxsci/kfh151
Hasselblatt P, Rath M, Komnenovic V et al (2007) Hepatocyte survival in acute hepatitis is due to c-Jun/AP-1-dependent expression of inducible nitric oxide synthase. Proc Natl Acad Sci USA 104:17105–17110. doi:10.1073/pnas.0706272104
Watanabe Y, Osaki H, Akaike T (1997) TNF-α bifunctionally induces proliferation in primary hepatocytes. Role of cell anchorage and spreading. J Immunol 159:4840–4847
Los M, Wesselborg S, Schulze-Osthoff K (1999) The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity 10:629–639. doi:10.1016/S1074-7613(00)80062-X
Slee EA, Harte MT, Kluck RM et al (1999) Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspase-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol 144:281–292. doi:10.1083/jcb.144.2.281
Urano F, Wang X, Bertolotti A et al (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666. doi:10.1126/science.287.5453.664
Nakagawa T, Zhu H, Morishima N et al (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403:98–103. doi:10.1038/47513
Ni M, Lee AS (2007) ER chaperones in mammalian development and human diseases. FEBS Lett 581:3641–3651. doi:10.1016/j.febslet.2007.04.045
Sokka A, Putkonen N, Mudo G et al (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 27:901–908. doi:10.1523/JNEUROSCI.4289-06.2007
Rubenstein RC, Zeitlin PL (2000) Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of ΔF508-CFTR. Am J Physiol Cell Physiol 278:C259–C267
Cnop M, Ladriere L, Hekerman P et al (2007) Selective inhibition of eukaryotic translation initiation factor 2α dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic β-cell dysfunction and apoptosis. J Biol Chem 282:3989–3997. doi:10.1074/jbc.M607627200
Ozcan U, Yilmaz E, Ozcan L et al (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140. doi:10.1126/science.1128294
Vilatoba M, Eckstein C, Bilbao G et al (2005) Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery 138:342–351. doi:10.1016/j.surg.2005.04.019
Acknowledgments
This work was supported by the High-Tech Research Center Project for Private Universities: matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology), 2004–2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanki, K., Kawamura, T. & Watanabe, Y. Control of ER stress by a chemical chaperone counteracts apoptotic signals in IFN-γ-treated murine hepatocytes. Apoptosis 14, 309–319 (2009). https://doi.org/10.1007/s10495-009-0318-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-009-0318-x