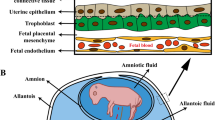
Abstract
The present review focuses on the physiological functions of glutamate–glutamine exchange involving placental amino acid transport and umbilical amino acid uptake in mammals (particularly in sows), with special emphasis on the associated regulating mechanisms. Glutamate plus glutamine are among the most abundant and the most utilized amino acids in fetus during late gestation. During pregnancy, amino acids, notably as precursors of macromolecules including proteins and nucleotides are involved in fetal development and growth. Amino acid concentrations in fetus are generally higher than in the mother. Among amino acids, the transport and metabolism of glutamate and glutamine during fetal development exhibit characteristics that clearly emphasize the importance of the interaction between the placenta and the fetal liver. Glutamate is quite remarkable among amino acids, which originate from the placenta, and is cleared from fetal plasma. In addition, the flux of glutamate through the placenta from the fetal plasma is highly correlated with the umbilical glutamate delivery rate. Glutamine plays a central role in fetal carbon and nitrogen metabolism and exhibits one of the highest fetal/maternal plasma ratio among all amino acids in human and other mammals. Glutamate is taken up by placenta from the fetal circulation and then converted to glutamine before being released back into the fetal circulation. Works are required on the glutamate–glutamine metabolism during late pregnancy in physiological and pathophysiological situations since such works may help to improve fetal growth and development both in humans and other mammals. Indeed, glutamine supplementation appears to ameliorate fetal growth retardation in sows and reduces preweaning mortality of piglets.




Similar content being viewed by others
Abbreviations
- BCAA:
-
Branched-chain amino acids
- CoA:
-
Coenzyme A
- CPS1:
-
Carbamyl phosphate synthetase 1
- GDM:
-
Gestational diabetes
- GS:
-
Glutamine synthetase
- IUGR:
-
Intrauterine growth restriction
- MSG:
-
Monosodium glutamate
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NAG:
-
N-acetylglutamate
- NAGS:
-
NAG synthase
- NCG:
-
N-carbamoylglutamate
- GDH:
-
Glutamate dehydrogenase
References
Andriamihaja M, Davila AM, Eklou-Lawson M, Petit N, Delpal S, Allek F, Blais A, Delteil C, Tomé D, Blachier F (2010) Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet. Am J Physiol 299:G1030–G1037
Avagliano L, Garo C, Marconi AM (2012) Placental amino acids transport in intrauterine growth restriction. J Pregnancy 2012:972562
Battaglia FC (2000) Glutamine and glutamate exchange between the fetal liver and the placenta. J Nutr 130:974S–977S
Blachier F, Guihot-Joubrel G, Vaugelade P, Le Boucher J, Bernard F, Duee P, Cynober L (1999) Portal hyperglutamatemia after dietary supplementation with monosodium glutamate in pigs. Digestion 60:349–357
Blachier F, Boutry C, Bos C, Tome D (2009) Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 90:814S–821S
Brosnan JT, Brosnan ME (2013) Glutamate: a truly functional amino acid. Amino Acids 45:413–418
Caldovic L, Ah Mew N, Shi D, Morizono H, Yudkoff M, Tuchman M (2010) N-acetylglutamate synthase: structure, function and defects. Mol Genet Metab 100(Suppl 1):S13–S19
Camelo JS Jr, Jorge SM, Martinez FE (2004) Amino acid composition of parturient plasma, the intervillous space of the placenta and the umbilical vein of term newborn infants. Braz J Med Biol Res 37:711–717
Campos PH, Silva BA, Donzele JL, Oliveira RF, Knol EF (2012) Effects of sow nutrition during gestation on within-litter birth weight variation: a review. Animal 6:797–806
Cetin I (2001) Amino acid interconversions in the fetal-placental unit: the animal model and human studies in vivo. Pediatr Res 49:148–154
Cetin I, Alvino G (2009) Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta 30(Suppl A):S77–S82
Cetin I, Ronzoni S, Marconi AM, Perugino G, Corbetta C, Battaglia FC, Pardi G (1996) Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am J Obstet Gynecol 174:1575–1583
Cetin I, de Santis MS, Taricco E, Radaelli T, Teng C, Ronzoni S, Spada E, Milani S, Pardi G (2005) Maternal and fetal amino acid concentrations in normal pregnancies and in pregnancies with gestational diabetes mellitus. Am J Obstet Gynecol 192:610–617
Cleal JK, Brownbill P, Godfrey KM, Jackson JM, Jackson AA, Sibley CP, Hanson MA, Lewis RM (2007) Modification of fetal plasma amino acid composition by placental amino acid exchangers in vitro. J Physiol 582:871–882
Day PE, Cleal JK, Lofthouse EM, Goss V, Koster G, Postle A, Jackson JM, Hanson MA, Jackson AA, Lewis RM (2013) Partitioning of glutamine synthesised by the isolated perfused human placenta between the maternal and fetal circulations. Placenta 34:1223–1231
Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M (2006) Programming placental nutrient transport capacity. J Physiol 572:5–15
Fowden AL, Sferruzzi-Perri AN, Coan PM, Constancia M, Burton GJ (2009) Placental efficiency and adaptation: endocrine regulation. J Physiol 587:3459–3472
Gao K, Jiang Z, Lin Y, Zheng C, Zhou G, Chen F, Yang L, Wu G (2012) Dietary l-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids 42:2207–2214
Gottlieb M, Wang Y, Teichberg VI (2003) Blood-mediated scavenging of cerebrospinal fluid glutamate. J Neurochem 87:119–126
Haberle J, Gorg B, Rutsch F, Schmidt E, Toutain A, Benoist JF, Gelot A, Suc AL, Hohne W, Schliess F, Haussinger D, Koch HG (2005) Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med 353:1926–1933
Heibel SK, Lopez GY, Panglao M, Sodha S, Marino-Ramirez L, Tuchman M, Caldovic L (2012) Transcriptional regulation of N-acetylglutamate synthase. PLoS One 7:e29527
Ivorra C, Garcia-Vicent C, Chaves FJ, Monleon D, Morales JM, Lurbe E (2012) Metabolomic profiling in blood from umbilical cords of low birth weight newborns. J Transl Med 10:142
Jones HN, Powell TL, Jansson T (2007) Regulation of placental nutrient transport–a review. Placenta 28:763–774
Jozwik M, Pietrzycki B, Jozwik M, Anthony RV (2009) Expression of enzymes regulating placental ammonia homeostasis in human fetal growth restricted pregnancies. Placenta 30:607–612
Kovacevic Z, McGivan JD (1983) Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev 63:547–605
Larque E, Ruiz-Palacios M, Koletzko B (2013) Placental regulation of fetal nutrient supply. Curr Opin Clin Nutr Metab Care 16:292–297
Lewis RM, Brooks S, Crocker IP, Glazier J, Hanson MA, Johnstone ED, Panitchob N, Please CP, Sibley CP, Widdows KL, Sengers BG (2013) Review: Modelling placental amino acid transfer–from transporters to placental function. Placenta 34(Suppl):S46–S51
Lin G, Liu C, Feng C, Fan Z, Dai Z, Lai C, Li Z, Wu G, Wang J (2012) Metabolomic analysis reveals differences in umbilical vein plasma metabolites between normal and growth-restricted fetal pigs during late gestation. J Nutr 142:990–998
Lin G, Wang X, Wu G, Feng C, Zhou H, Li D, Wang J (2014) Improving amino acid nutrition to prevent intrauterine growth restriction in mammals. Amino Acids. doi:10.1007/s00726-00014-01725-z
Liu XD, Wu X, Yu-long Y, Ya-qian L, Huan-sheng Y, Tie-jun L, Rui-lin H (2011) Effects of different dietary N-carbamylglutamate supplementation on the reproductive performance of sows during late pregnancy. Acta Veterinaria et Zootechnica Sinica 42:1550–1555
Liu XD, Wu X, Yin YL, Liu YQ, Geng MM, Yang HS, Blachier F, Wu GY (2012) Effects of dietary l-arginine or N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids 42:2111–2119
Liu C, Lin G, Wang X, Wang T, Wu G, Li D, Wang J (2013) Intrauterine growth restriction alters the hepatic proteome in fetal pigs. J Nutr Biochem 24:954–959
Marc Rhoads J, Wu G (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
Mateo RD, Wu G, Bazer FW, Park JC, Shinzato I, Kim SW (2007) Dietary l-arginine supplementation enhances the reproductive performance of gilts. J Nutr 137:652–656
Moores RR Jr, Vaughn PR, Battaglia FC, Fennessey PV, Wilkening RB, Meschia G (1994) Glutamate metabolism in fetus and placenta of late-gestation sheep. Am J Physiol 267:R89–R96
Murphy C, Newsholme P (1997) Glutamine as a possible precursor of l-arginine and thus nitric oxide synthesis in murine macrophages. Biochem Soc Trans 25:404S
Nakamura E, Torii K, Uneyama H (2008) Physiological roles of dietary free glutamate in gastrointestinal functions. Biol Pharm Bull 31:1841–1843
Neu J (2001) Glutamine in the fetus and critically ill low birth weight neonate: metabolism and mechanism of action. J Nutr 131:2585S–2589S (discussion 2590S)
Newsholme P, Lima MM, Procopio J, Pithon-Curi TC, Bazotte RB, Curi R (2003a) Glutamine and glutamate as vital metabolites. Braz J Med Biol Res 36:153–163
Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R (2003b) Glutamine and glutamate–their central role in cell metabolism and function. Cell Biochem Funct 21:1–9
Regnault TR, de Vrijer B, Battaglia FC (2002) Transport and metabolism of amino acids in placenta. Endocrine 19:23–41
Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G (2013) Umbilical uptakes and transplacental concentration ratios of amino acids in severe fetal growth restriction. Pediatr Res 73:602–611
Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, Fielder SE, Eide SJ, Lovering SL, Wu G (2013) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Salvolini E, Vignini A, Nanetti L, Raffaelli F, Di Primio R, Mazzanti L, Tranquilli AL (2012) Glutamate in vitro effects on human term placental mitochondria. J Matern Fetal Neonatal Med 25:952–956
Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C (2011) Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol 9:110
Sawant OB, Ramadoss J, Hankins GD, Wu G, Washburn SE (2014) Effects of l-glutamine supplementation on maternal and fetal hemodynamics in gestating ewes exposed to alcohol. Amino Acids 46:1981–1996
Self JT, Spencer TE, Johnson GA, Hu J, Bazer FW, Wu G (2004) Glutamine synthesis in the developing porcine placenta. Biol Reprod 70:1444–1451
Stegink LD, Pitkin RM, Reynolds WA, Filer LJ Jr, Boaz DP, Brummel MC (1975) Placental transfer of glutamate and its metabolites in the primate. Am J Obstet Gynecol 122:70–78
Timmerman M, Teng C, Wilkening RB, Fennessey P, Battaglia FC, Meschia G (2000) Effect of dexamethasone on fetal hepatic glutamine-glutamate exchange. Am J Physiol Endocrinol Metab 278:E839–E845
Tomlinson C, Rafii M, Sgro M, Ball RO, Pencharz P (2011) Arginine is synthesized from proline, not glutamate, in enterally fed human preterm neonates. Pediatr Res 69:46–50
Town SC, Patterson JL, Pereira CZ, Gourley G, Foxcroft GR (2005) Embryonic and fetal development in a commercial dam-line genotype. Anim Reprod Sci 85:301–316
Tujioka K, Fukaya Y, Sano A, Hayase K, Yokogoshi H (2005) Role of N-acetylglutamate turnover in urea synthesis of rats given proteins of different quality. J Nutr Sci Vitaminol Tokyo 51:93–98
van der Linden DS, Sciascia Q, Sales F, McCoard SA (2013) Placental nutrient transport is affected by pregnancy rank in sheep. J Anim Sci 91:644–653
Vaughn PR, Lobo C, Battaglia FC, Fennessey PV, Wilkening RB, Meschia G (1995) Glutamine-glutamate exchange between placenta and fetal liver. Am J Physiol 268:E705–E711
Washburn SE, Sawant OB, Lunde ER, Wu G, Cudd TA (2013) Acute alcohol exposure, acidemia or glutamine administration impacts amino acid homeostasis in ovine maternal and fetal plasma. Amino Acids 45:543–554
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2013) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G, Ott TL, Knabe DA, Bazer FW (1999) Amino acid composition of the fetal pig. J Nutr 129:1031–1038
Wu G, Knabe DA, Kim SW (2004) Arginine nutrition in neonatal pigs. J Nutr 134:2783S–2790S (discussion 2796S–2797S)
Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Li XL, Satterfield MC, Spencer TE (2010) Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci 88:E195–E204
Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL, Wang JJ (2011) Triennial Growth Symposium: important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu X, Yin YL, Liu YQ, Liu XD, Liu ZQ, Li TJ, Huang RL, Ruan Z, Deng ZY (2012) Effect of dietary arginine and N-carbamoylglutamate supplementation on reproduction and gene expression of eNOS, VEGFA and PlGF1 in placenta in late pregnancy of sows. Anim Reprod Sci 132:187–192
Wu G, Bazer FW, Johnson GA, Roberts JK, Li X, Dai Z (2013a) Maternal and fetal amino acid metabolism in gestating sows. Soc Reprod Fertil 68(Suppl):185–198
Wu G, Bazer FW, Satterfield MC, Li X, Wang X, Johnson GA, Burghardt RC, Dai Z, Wang J, Wu Z (2013b) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Wu G, Wu Z, Dai Z, Yang Y, Wang W, Liu C, Wang B, Wang J, Yin Y (2013c) Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 44:1107–1113
Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annual Rev Animal Biosci 2:387–417
Yamauchi K, Komatsu T, Kulkarni AD, Ohmori Y, Minami H, Ushiyama Y, Nakayama M, Yamamoto S (2002) Glutamine and arginine affect Caco-2 cell proliferation by promotion of nucleotide synthesis. Nutrition 18:329–333
Yu T, Zhao Y, Shi W, Ma R, Yu L (1997) Effects of maternal oral administration of monosodium glutamate at a late stage of pregnancy on developing mouse fetal brain. Brain Res 747:195–206
Acknowledgments
The writing of this review paper was jointly supported by grants from the NSFC (31110103909, 31101730), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2012BAD39B00), Chinese Academy of Sciences and Knowledge Innovation Project (CXJQ120113-1, 2012B091100259), China Postdoctoral Science Foundation funded project (2014M552022). Francois Blachier is a visiting professor granted by the Chinese Academy of Sciences (Grant no 2013T2S0014).
Conflict of interest
None of the authors have any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, X., Xie, C., Zhang, Y. et al. Glutamate–glutamine cycle and exchange in the placenta–fetus unit during late pregnancy. Amino Acids 47, 45–53 (2015). https://doi.org/10.1007/s00726-014-1861-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1861-5