Abstract
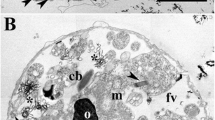
Trichocyst-enriched fractions were isolated from the marine dinophyte Prorocentrum micans. Transmission electron microscopy revealed that most of the trichocysts were discharged and had elongated to long filaments. Some trichocysts were still condensed. Fragments of discharged trichocysts measured up to 20 μm in length and 260 nm in width, those still condensed measured up to 1 μm in width and 16 μm in length. A distinct banding pattern with a transversal periodicity of approximately 16–18 nm and a periodic longitudinal striation of 3–4 nm could be measured along the trichocyst filaments. At higher magnifications, a fragile, alveolated, net-like organisation became obvious which resembled the one shown for the trichocysts of ciliates. When trichocyst-enriched fractions were treated with sodium dodecyl sulfate and centrifuged subsequently, no trichocysts were registered any longer in the sodium dodecyl sulfate-insoluble fraction by electron microscopy. Sodium dodecyl sulfate polyacrylamide gel electrophoresis of trichocyst-enriched fractions and of the SDS-soluble fractions revealed a protein banding pattern which was dominated by polypeptides of 50–30, 12.5, and approximately 8.5 kDa. The polypeptide banding pattern deviated significantly from those registered for ejectisomes of cryptophytes and of the prasinophyte Pyramimonas grossii, for the Reb polypeptides which constitute the R-bodies of Caedibacter taeniospiralis, and also from the banding pattern of trichocysts of Paramecium. An antiserum directed against trichocysts of Paramecium did not cross-react with the polypeptides present in the trichocyst-enriched fraction of Prorocentrum micans.





Similar content being viewed by others
References
Ammermann S, Schneider T, Westermann M, Hillebrand H, Rhiel E (2013) Ejectisins: tough and tiny polypeptides are a major component of cryptophycean ejectisomes. Protoplasma 250:551–563
Bannister LH (1972) The structure of trichocysts in Paramecium caudatum. J Cell Sci 11:899–929
Bathke L, Rhiel E, Krumbein WE, Marquardt J (1999) Biochemical and immunochemical investigations on the light-harvesting system of the cryptophyte Rhodomonas sp.: evidence for a photosystem I specific antenna. Plant Biol 1:516–523
Bouck GB, Sweeney BM (1966) The fine structure and ontogeny of trichocysts in marine dinoflagellates. Protoplasma 61:205–223
Dodge JD (1971) Fine structure of the pyrrophyta. Bot Rev 37:481–508
Dodge JD (1973) The fine structure of algal cells. Academic, London
Eperon S, Peck RK (1988) Structural and biochemical characterization of isolated trichocysts of the ciliate Pseudomicrothorax dubius. J Protozool 35:280–286
Gautier M-C, Sperling L, Madeddu L (1996) Cloning and sequence analysis of genes coding for Paramecium secretory granule (trichocyst) proteins. J Biol Chem 271:10247–10255
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervaceae (Cleve) Gran. Can J Microbiol 8:229–239
Hansen G (2001) Ultrastructure of Gymnodinium aureolum (Dinophyceae): toward a further redefinition of Gymnodinium sensu stricto. J Phycol 37:612–623
Hansen G, Moestrup Ø (1998) Light and electron microscopical observations on Peridiniella catenata (Dinophyceae). Eur J Phycol 33:293–305
Hausmann K (1973) Cytologische Studien an Trichocysten. VI. Feinstruktur und Funktionsmodus der Trichocysten der Flagellaten Oxyrrhis marina und des Ciliaten Pleuronema marinum. Helgoländer Meeresun 25:39–62
Hausmann K (1978) Extrusive organelles in protists. Int Rev Cytol 52:197–276
Hausmann K, Mignot J-P (1975) Cytologische Studien an Trichocysten. X. Die zusammengesetzten Trichocysten von Drepanomonas dentata Fresenius 1858. Protoplasma 83:61–78
Honsell G, Bonifacio A, De Bortoli M, Penna A, Battocchi C, Ciminiello P, Dell’Aversano C, Fattorusso E, Sosa S, Yasumoto T, Tubaro A (2013) New insights on cytological and metabolic features of Ostreopsis cf. ovata Fukuyo (Dinophyceae): a multidisciplinary approach. PLoS One 8(2):e57291
Hoppenrath M, Leander BS (2007) Morphology and phylogeny of the pseudocolonial dinoflagellate Polykrikos lebourae and Polykrikos herdmanae n. sp. Protist 158:209–227
Hoppenrath M, Saldarriaga JF (2012) Dinoflagellates. Version 15 December 2012 (under construction). http://tolweb.org/Dinoflagellates/2445/2012.12.15. In: The Tree of Life Web Project, http://tolweb.org/
Hoppenrath M, Yubuki N, Bachvaroff TR, Leander BS (2010) Re-classification of Phaeopolykrikos hartmannii as Polykrikos (Dinophyceae) based partly on the ultrastructure of complex extrusomes. Eur J Protistol 46:29–37
Hoppenrath M, Chomérat N, Horiguchi T, Schweikert M, Nagahama Y, Murray S (2013) Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae)—a proposal and review. Harmful Algae 27:1–28
Hwang JS, Nagai S, Hayakawa S, Takaku Y, Gojobori T (2008) The search for the origin of cnidarian nematocysts in dinoflagellates. In: Pontarotti P (ed) Evolutionary Biology from Concept to Application. Springer-Verlag, Berlin Heidelberg, pp 135–152
Kugrens P, Lee RE, Corliss JO (1994) Ultrastructure, biogenesis, and functions of extrusive organelles in selected non-ciliate protists. Protoplasma 181:164–190
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage T7. Nature 227:680–685
Livolant F (1982) Dinoflagellate trichocyst ultrastructure. I—the shaft. Biol Cell 43:201–210
Manton I (1969) Tubular trichocysts in a species of Pyramimonas (P. grossii PARKE). Österr Bot Z 116:378–392
Messer G, Ben-Shaul Y (1971) Fine structure of trichocyst fibrils of the dinoflagellate Peridinium westii. J Ultrastruct Res 37:94–104
Peterson JB, Heuser JE, Nelson DL (1987) Dissociation and reassociation of trichocyst proteins: biochemical and ultrastructural studies. J Cell Sci 87:3–25
Rhiel E, Westermann M (2012) Isolation, purification and some ultrastructural details of discharged ejectisomes of cryptophytes. Protoplasma 249:107–115
Rhiel E, Westermann M, Steiniger F, Kirchhoff C (2013) Isolation and characterization of the ejectisomes of the prasinophyte Pyramimonas grossii. Protoplasma 250:1351–1361
Rosati G, Modeo L (2003) Extrusomes in ciliates: diversification, distribution, and phylogenetic implications. J Eukaryot Microbiol 50:383–402
Sperling L, Tardieu A, Gulik-Krzywicki T (1987) The crystal lattice of Paramecium trichocysts before and after exocytosis by X-ray diffraction and freeze-fracture electron microscopy. J Cell Biol 105:1649–1662
Taylor FJR (1990) Chapter 24: Phylum Dinoflagellata. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ, McKhann HI (eds) Handbook of Protoctista. Jones and Bartlett, Boston, pp 419–437
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354
Ukeles R, Sweeney BM (1969) Influence of dinoflagellate trichocysts and other factors on the feeding of Crassostrea virginica larvae on Monochrysis lutheri. Limnol Oceanogr 14(3):403–410
Westfall J, Bradbury PC, Townsend JW (1983) Ultrastructure of the dinoflagellate Polykrikos. I. Development of the nematocyst-taeniocyst complex and morphology of the site for extrusion. J Cell Sci 63:245–261
Yamada N, Terada R, Tanaka A, Horiguchi T (2013) Bispinodinium angelaceum gen. et sp. nov. (Dinophyceae), a new sand-dwelling dinoflagellate from the seafloor off Mageshima Island, Japan. J Phycol 49:555–569
Yamagishi T, Kai A, Kawai H (2012) Trichocyst ribbons of a cryptomonads are constituted of homologs of R-body proteins produced by the intracellular parasitic bacterium of Paramecium. J Mol Evol 74:147–157
Yoon EY, Kang NS, Jeong HJ (2012) Gyrodinium moestrupii n. sp., a new planktonic heterotrophic dinoflagellate from the coastal waters of Western Korea: morphology and ribosomal DNA gene sequence. J Eukaryot Microbiol 59:571–586
Acknowledgments
The authors express their gratitude to Silke Ammermann, Ute Friedrich, Edith Kieselhorst, and Susanne Linde for excellent technical assistance, to Dr. Martina Schrallhammer (University of Freiburg) and Dr. Martin Simon (Saarland University) for kindly providing us the recombinant R-body polypeptides, isolated trichocysts of Paramecium and an antiserum directed against them.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Reimer Stick
This paper is dedicated to Renate Kort on the occasion of her 65th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Micrographs showing thecal pores of Prorocentrum micans either by scanning electron microscopy (A, B) or freeze-etching (C, D). Trichocysts in the process of being protruded are visible in B and C. The trichocyst in C withstood the replica cleaning process with proteinase K and hot nitric acid and its residue is visible as black structure. The scale bars are indicated (TIFF 9094 kb)
Online Resource 2
A Cryo-TEM micrograph of an isolated discharged trichocyst of Prorocentrum micans. After FFT analysis (B), a longitudinal frequency at 3.8 nm and a transversal frequency at 16.0 nm were detected (encircled spots in B). The encircled FFT spots at 16 and 3.8 nm were filtered out and an inverse FFT was calculated for the two frequencies. The transversal periodicity is visualized in C by an overlay of A and the inverse FFT of the 16.0 nm spots. The longitudinal periodicity is visualized in D by an overlay of A and the inverse FFT of the 3.8 nm spots. The scale bar represents 100 nm (TIFF 11779 kb)
MPEG 1-formated movie of negative-stained trichocysts of Prorocentrum micans. The tilt series was obtained using the TEMography software packages Recorder, Composer and Visualizer kai (System in Frontier Inc., Tokyo, Japan). The specimen was tilted from −69.01° to 58.05° and the micrographs were taken at 1° intervals (total of 129 micrographs) (MPG 1158 kb)
Online Resource 4
Pictures of 3-D reconstructed trichocysts obtained using the TEMography software packages Recorder, Composer and Visualizer kai (System in Frontier Inc., Tokyo, Japan). The micrographs shown in A and C were turned and tilted in order to get front views of the square-shaped profiles of trichocyst filaments (marked with arrows in B and D) (TIFF 3963 kb)
Rights and permissions
About this article
Cite this article
Westermann, M., Steiniger, F., Gülzow, N. et al. Isolation and characterisation of the trichocysts of the dinophyte Prorocentrum micans . Protoplasma 252, 271–281 (2015). https://doi.org/10.1007/s00709-014-0675-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0675-3