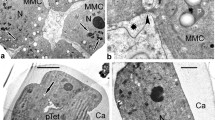
Summary.
Although the pollen grains produced in monocots are predominantly monosulcate (or monoporate), other aperture types are also found within this taxonomic group, such as the trichotomosulcate, inaperturate, zonaperturate, di-, or triaperturate types. The aperture pattern is determined during the young-tetrad stage of pollen development and it is known that some features of microsporogenesis can constrain the aperture type. For example, trichotomosulcate pollen is always associated with simultaneous cytokinesis, a condition considered as derived in the monocots. Our observations of the microsporogenesis pathway in a range of monocot species show that this pathway is surprisingly variable. Our results, however preliminary, reveal that variation in microsporogenesis concerns not only cytokinesis but also callose deposition among the microspores and shape of the tetrads. The role played by these features in aperture pattern determination is discussed.
Similar content being viewed by others
References
InstitutionalAuthorNameAngiosperm Phylogeny Group (2003) ArticleTitleAn update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II Bot J Linn Soc 141 399–436 Occurrence Handle10.1046/j.1095-8339.2003.t01-1-00158.x
K Arens (1949) ArticleTitleProva de Calose por meio da microscopia a luz fluorescente e aplicaçôes do metodo Lilloa 18 71–75
S Blackmore SH Barnes (1990) Pollen wall development in angiosperms S Blackmore RB Knox (Eds) Microspores: evolution and ontogeny Academic Press London 173–192
S Blackmore PR Crane (1998) The evolution of apertures in the spores and pollen grains of embryophytes SJ Owens PJ Rudall (Eds) Reproductive biology Royal Botanic Gardens Kew 159–182
RC Brown BE Lemmon (1988) ArticleTitleMicrotubules associated with simultaneous cytokinesis of coenocytic microsprocytes Am J Bot 75 1848–1856 Occurrence Handle10.2307/2444739
RC Brown BE Lemmon (1991) The cytokinetic apparatus in meiosis: control of division plane in the absence of a preprophase band of microtubules CW Lloyd (Eds) The cytoskeletal basis of plant growth and form Academic Press London 259–273
Buchner R, Weber M (2000) PalDat – a palynological database: descriptions, illustrations, identification, and information retrieval. http://paldat.botanik.univie.ac.at/
MW Chase DE Soltis PS Soltis PJ Rudall MF Fay WH Hahn S Sullivan J Joseph M Molvray PJ Kores TJ Givnish KJ Sytsma JC Pires (2000) Higher level systematics of the monocotyledons: an assessment of current knowledge and a new classification KL Wilson DA Morrison (Eds) Monocots: systematics and evolution Commonwealth Scientific and Industrial Research Organisation Sydney 3–16
G Erdtman (1952) Pollen morphology and plant taxonomy: angiosperms Almqvist and Wiksell Stockholm
CA Furness PJ Rudall (1999a) ArticleTitleInaperturate pollen in monocotyledons Int J Plant Sci 160 395–414 Occurrence Handle10.1086/314129
CA Furness PJ Rudall (1999b) ArticleTitleMicrosporogenesis in monocotyledons Ann Bot 84 475–499 Occurrence Handle10.1006/anbo.1999.0942
CA Furness PJ Rudall (2000) Aperture absence in pollen of monocotyledons MM Harley CM Morton S Blackmore (Eds) Pollen and spores: morphology and biology Royal Botanic Gardens Kew 249–257
P Goldblatt A Le Thomas (1993) ArticleTitlePollen morphology of Madagascan Aristea and Geosiris (Iridaceae-Nivenoideae) in relation to systematics and phylogeny Adansonia 14 223–233
P Goldblatt JC Manning PJ Rudall (1998) Iridaceae K Kubitzki (Eds) Flowering plants: monocotyledons SeriesTitleThe families and genera of vascular plants NumberInSeries3 Springer Berlin Heidelberg New York 295–335
MH Grayum (1992) ArticleTitleComparative external pollen ultrastructure of the Araceae and putatively related taxa Monogr Syst Not Missouri Bot Gard 43 1–167
MM Harley (1999a) Palm pollen: overview and examples of taxonomic value at species level A Henderson F Borchsenius (Eds) Evolution, variation, and classification of palms New York Botanical Garden Press Bronx, NY 95–120
MM Harley (1999b) Tetrad variation: its influence on pollen form and systematics in the Palmae MH Kurmann AR Hemsley (Eds) The evolution of plant architecture Royal Botanic Gardens Kew 289–304
MM Harley (2004) ArticleTitleTriaperturate pollen in the monocotyledons: configurations and conjectures Plant Syst Evol 247 75–122 Occurrence Handle10.1007/s00606-003-0107-x
MM Harley WJ Baker (2001) ArticleTitlePollen aperture morphology in Arecaceae: application within phylogenetic analyses, and a summary of the fossil record of palm-like pollen Grana 40 45–77 Occurrence Handle10.1080/00173130152591877
J Heslop-Harrison (1963) ArticleTitleAn ultrastructural study of pollen wall ontogeny in Silenependula Grana Palynol 4 7–24 Occurrence Handle10.1080/00173136309437854
K-L Huynh (1971) ArticleTitleEtude de l’arrangement du pollen dans la tétrade chez les Angiospermes sur la base de données cytologiques. III. Le pollen trilète du genre DianellaLam. (Liliaceae) Beitr Biol Pflanz 47 277–286
B Longly L Waterkeyn (1979a) ArticleTitleEtude de la cytocinèse. II. Structure et isolement des plaques cellulaires microsporocytaires Cellule 72 227–242
B Longly L Waterkeyn (1979b) ArticleTitleEtude de la cytocinèse. III. Les cloisonnements simultanés et successifs des microsporocytes Cellule 73 65–80
L Penet S Nadot A Ressayre A Forchioni LD Dreyer PH Gouyon (2005) ArticleTitleMultiple developmental pathways leading to a single morph: monosulcate pollen (examples from the Asparagales) Ann Bot 95 331–343 Occurrence Handle15567807 Occurrence Handle1:STN:280:DC%2BD2cnitVOruw%3D%3D
A Ressayre (2001) ArticleTitleEquatorial aperture pattern in monocots: same definition rules as in eudicots? The example of two species of Pontederiaceae Int J Plant Sci 162 1219–1224 Occurrence Handle10.1086/323477
A Ressayre B Godelle C Raquin P-H Gouyon (2002) ArticleTitleAperture pattern ontogeny in angiosperms J Exp Zool (Mol Dev Evol) 294 122–135 Occurrence Handle10.1002/jez.10150
A Ressayre A Mignot S Siljak-Yakovlev C Raquin (2003) ArticleTitlePostmeiotic cytokinesis and pollen aperture number determination in eudicots: effect of the cleavage wall number Protoplasma 221 257–268 Occurrence Handle12802633 Occurrence Handle1:STN:280:DC%2BD3s3nsFyhsg%3D%3D
A Ressayre LD Dreyer S Triki-Teurtroy A Forchioni S Nadot (2005) ArticleTitlePost-meiotic cytokinesis and pollen aperture pattern ontogeny: comparison of development in four species differing in aperture pattern Am J Bot 92 576–583
JL Roth JW Walker AG Walker (1987) ArticleTitleThe distribution and systematics of trichotomosulcate pollen within the Lilialean complex Am J Bot 74 751
PJ Rudall CA Furness MW Chase MF Fay (1997) ArticleTitleMicrosporogenesis and pollen sulcus type in Asparagales (Lilianae) Can J Bot 75 408–430
K Schnarf R Wunderlich (1939) ArticleTitleZur vergleichenden Embryologie der Liliaceae: Asphodeloideae Flora 133 297–327
JM Sheldon HG Dickinson (1983) ArticleTitleDetermination of patterning in the pollen wall of Lilium henryi J Cell Sci 63 191–208 Occurrence Handle6313711 Occurrence Handle1:STN:280:DyaL2c%2Fjt1Ohtg%3D%3D
JM Sheldon HG Dickinson (1986) ArticleTitlePollen wall formation in Lilium: the effect of chaotropic agents and the organization of the microtubular cytoskeleton during pattern development Planta 168 11–23 Occurrence Handle1:CAS:528:DyaL28Xktl2nu70%3D Occurrence Handle10.1007/BF00407003
F Stainier D Huard F Bronkers (1967) ArticleTitleTechnique de coloration spécifique de l’exine des microspores jeunes encore groupées en tétrade Pollen Spores 9 367–370
I Till-Bottraud A Mignot R De Paepe I Dajoz (1995) ArticleTitlePollen heteromorphism in Nicotiana tabacum (Solanaceae) Am J Bot 82 1040–1048 Occurrence Handle10.2307/2446234
RP Wodehouse (1935) Pollen grains McGraw Hill New York
Author information
Authors and Affiliations
Corresponding author
Additional information
Correspondence and reprints: Laboratoire Ecologie, Systématique et Evolution, Université Paris-Sud, 91405 Orsay Cedex, France.
Rights and permissions
About this article
Cite this article
Nadot, S., Forchioni, A., Penet, L. et al. Links between early pollen development and aperture pattern in monocots. Protoplasma 228, 55–64 (2006). https://doi.org/10.1007/s00709-006-0164-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-006-0164-4