Summary.
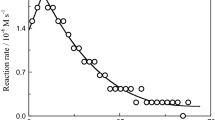
The kinetics of the formation of the 1:3 complex of chromium(III) with L-glutamic acid and DL-lysine were studied spectrophotometrically at and 550 nm. The reaction was found to be first order in both reactants. Increasing the hydrogen ion concentration from 3.2×10−5 to 1.0×10−3 molċdm−3 retarded the reaction rate which is of the form . Values of 28.8 and 63.6 kJċmol−1 were obtained for the energy of activation and −184 and −116 Jċ K−1ċmol−1 for the entropy of activation for L-glutamic acid and DL-lysine. The logarithms of the formation constants of the two complexes were found to be 5.9 and 5.1.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received January 7, 2000. Accepted (revised) March 8, 2000
Rights and permissions
About this article
Cite this article
Guindy, N., Abou-Gamra, Z. & Abdel-Messih, M. Kinetic Studies on the Complexation of Chromium(III) with some Amino Acids in Aqueous Acidic Medium. Monatshefte fuer Chemie 131, 857–866 (2000). https://doi.org/10.1007/s007060070063
Issue Date:
DOI: https://doi.org/10.1007/s007060070063