Abstract
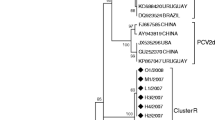
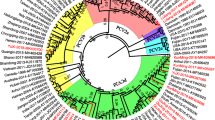
Porcine circovirus type 2 (PCV2) is the cause of postweaning multisystemic wasting syndrome (PMWS), which encompasses several distinct symptoms in pigs. PCV2 infection and clinical incidence of PMWS have increased in recent years, possibly due to shifts in viral populations and mutations. In this study, we identified PVC2 strains currently afflicting pig populations in mainland China, because this is a prerequisite for developing a specific vaccine to control the spread of PMWS. We collected 235 tissue samples from 16 provinces between 2014 and 2016. Of these, 152 samples were positive for PCV2. We compared the sequences we obtained for the PVC2 capsid gene, ORF2, to those of the Chinese PCV2 sequences deposited in GenBank between 2002 and 2016 (n = 648). Phylogenetic analyses demonstrated that the PCV2d genotype was the most prevalent strain in the sample population included in GenBank and among the positive samples from this study. We also found one PCV2c strain among the GenBank sequences. Furthermore, PCV2a-2F was the predominant genotype in the PCV2a cluster. Amino acid sequence comparisons demonstrated 70.8–100% identity within PCV ORF2 and several consistent mutations in ORF2. More interestingly, six isolates were classified as recombinant strains. Cumulatively, this study represents the first comprehensive description of PCV2 strains distribution, including recent samples, in Chinese porcine populations. We demonstrate the existence of high genetic variability among PVC2 strains and the ability of this virus to rapidly evolve.




Similar content being viewed by others
References
Allan GM, Mc Neilly F, Meehan BM, Kennedy S, Mackie DP, Ellis JA, Clark EG, Espuna E, Saubi N, Riera P, Botner A, Charreyre CE (1999) Isolation and characterisation of circoviruses from pigs with wasting syndromes in Spain, Denmark and Northern Ireland. Vet Microbiol 66:115–123
Allan GM, McNeilly E, Kennedy S, Meehan B, Moffett D, Malone F, Ellis J, Krakowka S (2000) PCV-2-associated PDNS in Northern Ireland in 1990. Porcine dermatitis and nephropathy syndrome. Vet Rec 146:711–712
Anoopraj R, Rajkhowa TK, Cherian S, Arya RS, Tomar N, Gupta A, Ray PK, Somvanshi R, Saikumar G (2015) Genetic characterisation and phylogenetic analysis of PCV2 isolates from India: indications for emergence of natural inter-genotypic recombinants. Infect Genet Evol 31:25–32
Cai L, Han X, Ni J, Yu X, Zhou Z, Zhai X, Chen X, Tian K (2011) Natural recombinants derived from different patterns of recombination between two PCV2b parental strains. Virus Res 158:281–288
Choi KS, Chae JS (2008) Genetic characterization of porcine circovirus type 2 in Republic of Korea. Res Vet Sci 84:497–501
Cortey M, Olvera A, Grau-Roma L, Segales J (2011) Further comments on porcine circovirus type 2 (PCV2) genotype definition and nomenclature. Vet Microbiol 149:522–523
Ellis J, Hassard L, Clark E, Harding J, Allan G, Willson P, Strokappe J, Martin K, McNeilly F, Meehan B, Todd D, Haines D (1998) Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can Vet J 39:44–51
Fenaux M, Halbur PG, Gill M, Toth TE, Meng XJ (2000) Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J Clin Microbiol 38:2494–2503
Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ (2004) Two amino acid mutations in the capsid protein of type 2 porcine circovirus (PCV2) enhanced PCV2 replication in vitro and attenuated the virus in vivo. J Virol 78:13440–13446
Franzo G, Cortey M, de Castro AM, Piovezan U, Szabo MP, Drigo M, Segales J, Richtzenhain LJ (2015) Genetic characterisation of Porcine circovirus type 2 (PCV2) strains from feral pigs in the Brazilian Pantanal: An opportunity to reconstruct the history of PCV2 evolution. Vet Microbiol 178:158–162
Franzo G, Tucciarone CM, Dotto G, Gigli A, Ceglie L, Drigo M (2015) International trades, local spread and viral evolution: the case of porcine circovirus type 2 (PCV2) strains heterogeneity in Italy. Infect Genet Evol 32:409–415
Gagnon CA, Music N, Fontaine G, Tremblay D, Harel J (2010) Emergence of a new type of porcine circovirus in swine (PCV): a type 1 and type 2 PCV recombinant. Vet Microbiol 144:18–23
Ge X, Wang F, Guo X, Yang H (2012) Porcine circovirus type 2 and its associated diseases in China. Virus Res 164:100–106
Guo L, Lu Y, Wei Y, Huang L, Wu H, Liu C (2011) Porcine circovirus genotype 2a (PCV2a) and genotype 2b (PCV2b) recombinant mutants showed significantly enhanced viral replication and altered antigenicity in vitro. Virology 419:57–63
Guo LJ, Lu YH, Wei YW, Huang LP, Liu CM (2010) Porcine circovirus type 2 (PCV2): genetic variation and newly emerging genotypes in China. Virol J 7:273
Haldimann A, Nicolet J, Frey J (1993) DNA sequence determination and biochemical analysis of the immunogenic protein P36, the lactate dehydrogenase (LDH) of Mycoplasma hyopneumoniae. J Gen Microbiol 139:317–323
Huang Y, Shao M, Xu X, Zhang X, Du Q, Zhao X, Zhang W, Lyu Y, Tong D (2013) Evidence for different patterns of natural inter-genotype recombination between two PCV2 parental strains in the field. Virus Res 175:78–86
Hurtado A, Garcia-Perez AL, Aduriz G, Juste RA (2003) Genetic diversity of ruminant pestiviruses from Spain. Virus Res 92:67–73
Jaganathan S, Toung OP, Yee PL, Yew TD, Yoon CP, Keong LB (2011) Genetic characterization of porcine circovirus 2 found in Malaysia. Virol J 8:437
Lekcharoensuk P, Morozov I, Paul PS, Thangthumniyom N, Wajjawalku W, Meng XJ (2004) Epitope mapping of the major capsid protein of type 2 porcine circovirus (PCV2) by using chimeric PCV1 and PCV2. J Virol 78:8135–8145
Li W, Wang X, Ma T, Feng Z, Li Y, Jiang P (2010) Genetic analysis of porcine circovirus type 2 (PCV2) strains isolated between 2001 and 2009: genotype PCV2b predominate in postweaning multisystemic wasting syndrome occurrences in eastern China. Virus Genes 40:244–251
Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC (1999) Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 73:152–160
Ma CM, Hon CC, Lam TY, Li VY, Wong CK, de Oliveira T, Leung FC (2007) Evidence for recombination in natural populations of porcine circovirus type 2 in Hong Kong and mainland China. J Gen Virol 88:1733–1737
Martin DP, Lemey P, Lott M, Moulton V, Posada D, Lefeuvre P (2010) RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26:2462–2463
Gomes Martins, de Castro AM, Cortez A, Heinemann MB, Brandao PE, Richtzenhain LJ (2007) Genetic diversity of Brazilian strains of porcine circovirus type 2 (PCV-2) revealed by analysis of the cap gene (ORF-2). Arch Virol 152:1435–1445
Molitor TW, Oraveerakul K, Zhang QQ, Choi CS, Ludemann LR (1991) Polymerase chain reaction (PCR) amplification for the detection of porcine parvovirus. J Virol Methods 32:201–211
Mu CL, Yang QY, Zhang YN, Zhou YH, Zhang JX, Martin DP, Xia PA, Cui BA (2012) Genetic variation and phylogenetic analysis of porcine circovirus type 2 infections in central China. Virus Genes 45:463–473
Olvera A, Cortey M, Segales J (2007) Molecular evolution of porcine circovirus type 2 genomes: phylogeny and clonality. Virology 357:175–185
Opriessnig T, Halbur PG (2012) Concurrent infections are important for expression of porcine circovirus associated disease. Virus Res 164:20–32
Opriessnig T, Xiao CT, Gerber PF, Halbur PG (2014) Identification of recently described porcine parvoviruses in archived North American samples from 1996 and association with porcine circovirus associated disease. Vet Microbiol 173:9–16
Opriessnig T, O’Neill KGP, de Castro AM, Gimenéz-Lirola LG, Beach NM, Zhou L, Meng XJ, Wang C, Halbur PG (2013) A PCV2 vaccine based on genotype 2b is more effective than a 2a-based vaccine to protect against PCV2b or combined PCV2a/2b viremia in pigs with concurrent PCV2, PRRSV and PPV infection. Vaccine 31:487–494
Pogranichnyy RM, Yoon KJ, Harms PA, Swenson SL, Zimmerman JJ, Sorden SD (2000) Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral Immunol 13:143–153
Ramos N, Mirazo S, Castro G, Arbiza J (2013) Molecular analysis of Porcine Circovirus Type 2 strains from Uruguay: evidence for natural occurring recombination. Infect Genet Evol 19:23–31
Rodriguez-Carino C, Segales J (2009) Ultrastructural findings in lymph nodes from pigs suffering from naturally occurring postweaning multisystemic wasting syndrome. Vet Pathol 46:729–735
Rosell C, Segales J, Ramos-Vara JA, Folch JM, Rodriguez-Arrioja GM, Duran CO, Balasch M, Plana-Duran J, Domingo M (2000) Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome. Vet Rec 146:40–43
Stevenson GW, Kiupel M, Mittal SK, Choi J, Latimer KS, Kanitz CL (2001) Tissue distribution and genetic typing of porcine circoviruses in pigs with naturally occurring congenital tremors. J Vet Diagn Invest 13:57–62
Sun J, Huang L, Wei Y, Wang Y, Chen D, Du W, Wu H, Liu C (2015) Prevalence of emerging porcine parvoviruses and their co-infections with porcine circovirus type 2 in China. Arch Virol 160:1339–1344
Wang C, Huang TS, Huang CC, Tu C, Jong MH, Lin SY, Lai SS (2004) Characterization of porcine circovirus type 2 in Taiwan. J Vet Med Sci 66:469–475
Wang F, Guo X, Ge X, Wang Z, Chen Y, Cha Z, Yang H (2009) Genetic variation analysis of Chinese strains of porcine circovirus type 2. Virus Res 145:151–156
Wei CY, Zhang MZ, Chen Y, Xie JX, Huang Z, Zhu WJ, Xu TC, Cao ZP, Zhou P, Shuo SuS, Zhang GH (2013) Genetic evolution and phylogenetic analysis of porcine circovirus type 2 infections in southern China from 2011 to 2012. Infect Genet Evol 17:87–92
Xiao CT, Halbur PG, Opriessnig T (2015) Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J Gen Virol 96:1830–1841
Yang HC (2004) Epidemical characterizations and control strategies of swine immunosuppressive diseases (PRRS and PMWS). Chin J Anim Husband Vet Med 31:41–43
Yoon KJ, Zimmerman JJ, Chang CC, Cancel-Tirado S, Harmon KM, McGinley MJ (1999) Effect of challenge dose and route on porcine reproductive and respiratory syndrome virus (PRRSV) infection in young swine. Vet Res 30:629–638
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 31270045).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of this paper.
Rights and permissions
About this article
Cite this article
Jiang, CG., Wang, G., Tu, YB. et al. Genetic analysis of porcine circovirus type 2 in China. Arch Virol 162, 2715–2726 (2017). https://doi.org/10.1007/s00705-017-3414-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3414-1