Abstract
We have investigated whether poly(I:C) Toll-like receptor 3 (TLR3) and resiquimod Toll-like receptor 7 (TLR7) agonists can serve as vaccine adjuvants and promote the efficiency of therapeutic DNA vaccination against tumors expressing the human papilloma virus 16 (HPV-16) E7 protein. For this purpose, C57BL/6 mice were inoculated with 2 × 105 TC-1 cells, and they were then immunized with HPV-16 E7 DNA vaccine alone or with 50 μg of resiquimod or poly(I:C) individually. We found that poly(I:C) and resiquimod could induce more antigen-specific lymphocyte proliferation and cytolytic activity compared to vaccination with E7 DNA alone. While E7 DNA had no significant inhibitory effect on tumor growth, co-administration of poly(I:C) and resiquimod with E7 DNA induced significant tumor regression. Peripheral and local cytokine assays demonstrated that co-administration of poly(I:C) and resiquimod with E7 DNA induced circulating antigen-specific IFN-γ and nonspecific intratumoral IL-12. TLR3 and TLR7 agonists can be used to enhance the immune response to DNA vaccine immunogens. Taken together, these data indicate that combined vaccination with DNA encoding HPV-16 E7 plus TLR agonists provides a strategy for improving the efficacy of a vaccine as a possible immunotherapeutic strategy for cervical cancer.
Similar content being viewed by others
Introduction
Human papilloma viruses (HPVs) have been identified as the etiological agents of cervical cancer, the second most common malignancy in women worldwide [1]. The HPV oncoproteins E6 and E7 are consistently expressed in HPV-associated cancer cells and are responsible for their malignant transformation [23, 24]. Several lines of evidence suggest that cell-mediated immunity is important in controlling both HPV infection and HPV-associated neoplasms [35]. Therefore, HPV E6 and E7 are ideal target antigens for immunotherapy of HPV-induced lesions and tumors.
The use of DNA vaccines has become an attractive approach for the development of antigen-specific immunotherapy. The advantages of plasmid DNA immunization are its ability to induce T-helper 1 (Th1) and CTL responses, prolong antigen expression, and potentially induce long-lived effector activity [18, 20]. One of the concerns about DNA vaccines is their limited potency. Therefore, ongoing research aims to augment the responses to DNA vaccination through several mechanisms, including co-administration of novel immunostimulants and adjuvants [16, 17].
The use of adjuvants to improve vaccine efficacy is typically feasible and cost-effective. Currently, the functions of adjuvants are believed to be to activate innate immunity, to increase the interaction between antigens, and to modulate the intrinsic immunogenicity of an antigen [30]. Chemical agonists of Toll-like receptors (TLR) are now being considered as novel tools for screening potent adjuvants for vaccine development [27]. The use of these TLR agonists in vaccine formulations may permit the development of effective therapeutic vaccine strategies for the immunotherapy of cancer [3]. Several reports have shown improved efficiency of vaccines in mice when antigen delivery is combined with a TLR agonist [39].
Imiquimod and resiquimod (R848) are Toll-like receptor (TLR) 7 and 8 agonists, and they are currently being investigated as adjuvants in FDA-approved clinical trials [10, 11]. These adjuvants have been shown to have immune-response-modifying properties in vivo and to induce antiviral and anti-tumor activity as well as stimulating cytokine secretion in dendritic cells (DCs) [26]. Specifically, it has been demonstrated that resiquimod activates the innate immune system via TLR-7 through the activation of the MyD88 pathway in DCs. Activated DCs are thought to follow a set program in which they secrete inflammatory cytokines [14].
Polyinosinic-polycytidylic acid (poly(I:C)), a TLR3 agonist, is an artificial mimic of viral double-stranded RNA and an interferon (IFN) inducer [21], and it has long been known to be an antiviral agent. Toll-like receptor 3 promotes cross-priming to virus-infected cells [4, 34]. Poly(I:C) can also serve as an effective adjuvant for improving cellular immunity in response to vaccines [29, 32]. However, a detailed comparison of the efficiency of these vaccine adjuvants has not been done, and there have been no comparative studies in the DNA vaccine area. Therefore, we compared the effects of co-administration of resiquimod or poly(I:C) individually as Toll-like receptor agonists with an HPV-16 E7 DNA vaccine and conclude that the DNA vaccine in combination with resiquimod or poly(I:C) induced a strong specific immune response with a bias towards a Th1 immune profile. These combinations also induced antitumor responses in a tumor microenvironment by increasing the level of pro-inflammatory T helper 1 (Th1)-related cytokines and decreasing the level of IL-10, a key factor required for tumor growth.
Materials and methods
Mice and cells
Female 6- to 8-week-old C57BL/6 mice were obtained from the Institute Pasteur of Iran (Karaj, Iran). Mice were housed for 1 week before the experiment, given free access to food and water, and maintained in a light/dark cycle. All experiments were carried out in accordance with the Animal Care and Use Protocol of Golestan University of Medical Sciences of Iran.
The production and maintenance of TC-1 cells have been described previously [13]. TC-1 cells were grown in RPMI 1640, supplemented with 10 % (v/v) fetal bovine serum, 50 units of penicillin/streptomycin per mL, 2 mM glutamine, 1 mM sodium pyruvate, 2 mM nonessential amino acids, and 0.4 mg G418 per mL at 37 °C with 5 % CO2.
DNA vaccine and adjuvants
The generation of pcDNA3-E7 has been described previously. Plasmid constructs were confirmed by DNA sequencing and expression. Amplification and purification of DNA were described previously [12].
Resiquimod (1-(2-hydroxy-2-methylpropyl)-2-methyl-1H-imidazo[4,5-c]quinolin-4-amine), formerly R-848) and agonists of TLR3 (poly(I:C)) (InvivoGen) were dissolved in PBS at a final concentration of 1 μg/ml and were administered at a dose of 50 μg per subcutaneous (S.C.) injection. PBS was administered as negative control using the same schedule.
In vivo tumor treatment experiment
For in vivo therapeutic experiments, C57BL/6 mice were divided into five groups (n = 10). The mice were challenged by subcutaneous (S.C.) injection in the right flank with 2 × 105 TC-1 cells suspended in 100 μl PBS. After one week, the mice were immunized S.C. in the right flank with 90 μg of DNA vaccine encoding HPV-16 E7 three times at 7-day intervals (group 1). In two groups, the poly(I:C)-E7 DNA and resiquimod-E7 DNA, resiquimod and poly(I:C) were dissolved in 100 μl PBS containing 90 μg of the pcDNA3-E7 construct and injected to each mouse, using the same protocol, i.e., resiquimod or poly(I:C) was administered S.C. in combination with pcDNA3-E7 three times at 7-day intervals (resiquimod-E7 and poly(I:C)-E7 groups). PBS and pcDNA3 were injected according to the same protocol into the fourth and fifth groups of mice as negative controls (PBS and pcDNA3 groups).
Subcutaneous tumor volume was estimated according to Carlsson’s formula [13]. Hence, the largest (a) and the smallest (b) superficial diameters of the tumor were measured in a blinded, coded fashion twice a week, and then the volume (V) of the tumor was calculated (V = a × b × b/2). Statistical analysis was performed using Student’s t-test. All values were expressed as mean ± S.D.
Three mice per group were sacrificed one week following the third immunization, and the spleens were removed aseptically. Cell proliferation, cytolytic activity and cytokine secretion were then assayed. All tests were performed in triplicate for each mouse. Results are representative of three independent experiments.
Lymphocyte proliferation assay (LPA)
One week after the third immunization, three mice per group were sacrificed, and their splenocytes were isolated and treated with ammonium chloride-potassium lysing buffer for 1 min to deplete erythrocytes. In 96-well flat-bottom culture plates (Nunc, Denmark) spleen cells (2 × 105 per well) were cultured in triplicate with RPMI-1640 supplemented with 10 % fetal calf serum, 1 % L-glutamine, 1 % HEPES, and 0.1 % penicillin/streptomycin and incubated in the presence of E7-specific H-2Db CTL epitope at a concentration of 1 μg/ml (specific antigen), T cell mitogen PHA (phytohemagglutinin, positive control), 2 μg of BSA per ml (irrelevant antigen) or medium (negative control) at 37 °C in 5 % CO2. After 3 days, MTT (3-(4,5-dimethyl tetrazolyl-2) 2,5 diphenyltetrazolium bromide (Sigma Chemicals) at a concentration of 5 μg/ml was added to each well and incubated for 5 h at 37 °C in 5 % CO2. DMSO (dimethyl sulfoxide) (100 μl) was added to dissolve formazan crystals that were produced.
Plates were read at 540 nm, and the results were expressed as a stimulation index (SI). The SI was determined as follows: OD value of stimulated cells (Cs) minus relative number of unstimulated cells (Cu) divided by the relative OD value of unstimulated cells.
All tests were performed in triplicate for each mouse.
CTL assay
One week after the third immunization, for each sample obtained from an individual mouse (three mice per group), a single cell suspension of mononuclear cells (used as the effecter cells) was cocultured in RPMI 1640 medium with washed EL4 target cells at a 50:1 effector-to-target cell (E/T) ratio, at which maximal release of LDH was observed. For preparation of the target cells, EL-4 cells were stimulated with E7-specific H-2Db CTL epitope (E7, amino acids 49–57) at a concentration of 1 μg/ml and then incubated for 4 h.
After centrifugation, the supernatants (50 μl/well) were transferred to 96-well flat-bottom plates, and lysis of target cells were determined by measuring LDH release using a Cytotoxicity Detection Kit (LDH) according to the procedure recommended by the manufacturer (Takara). Several controls were used for the cytotoxicity assay.
“High control” was the total LDH released from the target cells when all EL4 cells were lysed with medium containing 1 % Triton X-100. “Low control” was the natural release of LDH from the target cells, which was obtained by adding only EL4 cells to the assay medium. “T-cell control” was used to measure the natural release of LDH from T cells and was obtained by adding only different numbers of T cells to the assay medium. The assay for all samples, including the controls, was performed in triplicate.
The LDH-mediated conversion of the tetrazolium salt into the red formazan product was measured at 490 nm after incubation at room temperature for 30 min. The percentage of specific cytolysis was determined by the following formula:
Systemic cytokine assay
One week after the third immunization, mononuclear cells from spleens of immunized mice at a concentration of 2 × 106 cells/well in 24-well plates (Nunc, Denmark) were incubated for 2 days in a total volume of 1.5 ml of RPMI-1640 supplemented with 10 % FCS, 1 % L-glutamine, 1 % HEPES, 0.1 % 2ME, 0.1 % penicillin/streptomycin and pulsed with E7-specific epitope (E7, amino acids 49–57) at a concentration of 1 μg/ml at 37 °C in 5 % CO2. The cell supernatants were collected and assayed for the presence of IFN-γ and IL-4 using commercially available sandwich-based ELISA kits (eBioscience, Inc., San Diego, CA) following the manufacturer’s instructions. All tests were performed in triplicate for each mouse (three mice per group).
Intratumoral cytokine assay
Twenty-four hours after the last administration, TC-1 tumors were harvested and weighed, minced into small pieces, and sonicated for 30 s in lysis buffer (PBS with 1 % Triton-X, 1 mM phenylmethanesulfonyl fluoride [PMSF]: 1 mL of lysis buffer per 100 mg tumor). The samples then were homogenized and centrifuged. The levels of IL-12 and IL-10 in tumor supernatants were measured using commercially available sandwich-based ELISA kits (eBioscience, Inc. San Diego, CA). The results are presented as the amount of cytokine (pg/mL) per 20 million tumor cells.
Statistical analysis
Lymphocyte proliferation, CTL and cytokine assay were analyzed by a one-way ANOVA. Significant differences in tumor growth on given days were assessed by Student’s t-test. Differences were considered statistically significant when the P-value was less than 0.05.
Results
The co-administration of resiquimod and poly(I:C) with E7 DNA vaccine enhances lymphocyte proliferation
To determine whether the Toll-like receptor agonist influenced the cell-mediated response, we performed antigen-specific T cell proliferation assay in vitro. Splenocytes were isolated from immunized mice, divided into four parts, and stimulated with HPV-16 E7-specific CTL epitope as a specific antigen, PHA as a positive control, BSA as an irrelevant control, and medium as a negative control.
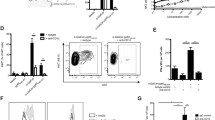
As shown in Fig. 1, lymphocyte proliferation was significantly higher in mice treated with the DNA vaccine, poly(I:C)-E7 DNA and resiquimod-E7 DNA than in the negative control groups (PBS and pcDNA 3) (P < 0.05). Furthermore, the poly(I:C)-E7 DNA and resiquimod-E7 DNA groups exhibited significantly higher lymphocyte proliferation than the group treated with E7 DNA without adjuvants (P < 0.01). The T cell proliferation response was highest in the mice immunized with resiquimod-DNA vaccine HPV-16 E7.
Splenocyte proliferation levels after in vitro stimulation with an HPV-16 E7 epitope. C57BL/6 mice were challenged by subcutaneous injection of TC-1 cells. After one week, the mice were immunized three times subcutaneously with poly(I:C)-E7 DNA, resiquimod-E7 DNA, E7 DNA (test groups), and pcDNA3, and PBS (negative control groups). One week after final immunization, spleens of individual mice (n = 3) were removed, and lymphocyte proliferation was evaluated by the MTT method. Formazan crystal formation after incubation with MTT was determined by dissolving the crystals in DMSO, and optical density (OD) was read at 540 nm. All tests were performed in triplicate for each mouse. Values are the mean ± SD of the mean for the experiments. Lymphocyte proliferation of the poly(I:C)-E7 DNA and resiquimod-E7 DNA groups was significantly higher than those in the control groups (P < 0.05). Results are representative of three independent experiments. ** Indicates statistically significant difference between the indicated groups as determined by one-way ANOVA (P < 0.01). The graph also shows the statistically significant differences between all treatment groups and the PBS and pcDNA3 control groups (P < 0.05)
The co-administration of resiquimod and poly(I:C) with E7 DNA vaccine enhances the CTL response
CTLs play a key role in the antitumor immune response against cancers. In our study, the CTL response in immunized mice was examined using the LDH release assay. As shown in Fig. 2, the cytolytic activity was significantly higher in mice treated with the DNA vaccine, the poly(I:C)-E7 DNA and resiquimod-E7 DNA groups than in the negative control groups (PBS and pcDNA 3) (P < 0.05). Furthermore, the poly(I:C)-E7 DNA and resiquimod-E7 DNA groups had a significantly higher antigen-specific CTL response than the group treated with E7 DNA without adjuvants (P < 0.001).
Comparison of cytotoxic activity in mice vaccinated with Toll-like receptor agonists as a DNA vaccine adjuvant. After mice (n = 3) were immunized three times with the vaccines, spleens were harvested as described in Materials and methods. Target cell lysis was quantitated using an LDH release assay and expressed as percent cytotoxicity ± SD. Results are representative of at least three independent experiments. *** Indicates a statistically significant difference between the indicated groups and those that received E7 DNA alone, as determined by one-way ANOVA (P < 0.001). The graph also shows the statistically significant differences between all treatment groups and the PBS and pcDNA3 control groups (P < 0.05)
The co-administration of poly(I:C) with E7 DNA vaccine shifts the cytokine pattern toward Th1, but resiquimod-E7 DNA induces a mixed Th1 and Th2 response profile
Cellular immune responses were also evaluated by cytokine secretion assay of the in vitro stimulated splenocytes from all vaccinated animals. IFN-γ secretion is a marker of Th1-type antigen-specific cellular immune responses and plays key roles in fighting cancer, while IL-4 is recognized as Th2 biased.
The cytokine profiles induced by vaccination with E7 DNA were investigated following co-administration of resiquimod and poly(I:C). As shown in Fig. 3, mice immunized with the DNA vaccine produced significantly more IFN- γ and IL-4 than PBS- and pcDNA3-immunized mice (P < 0.05). Both IFN- γ and IL-4 cytokines were increased when resiquimod and poly(I:C) were co-administered with E7 DNA. With resiquimod and poly(I:C), increases in IFN-γ (indicative of Th1 responses) were greater than those of IL-4 (indicative of Th2 responses). Lymphocytes from the poly(I:C)-E7 DNA and resiquimod-E7 DNA groups produced the largest amounts of IFN-γ. Furthermore, the combination of poly(I:C)-E7 DNA and resiquimod-E7 DNA vaccine stimulated IFN-γ production more than E7 DNA vaccine alone (P < 0.001). While the combination groups had similar levels of IFN-γ, the resiquimod-E7 DNA group produced significantly more IL-4 than mice immunized with the combination of poly(I:C)-E7 DNA and DNA vaccine alone (P < 0.001).
Determination of the ratios of TH1/TH2 cytokines by enzyme-linked immunosorbent assay (ELISA). Collected supernatants (n = 3) from stimulated splenocytes were screened for the presence of IFN-γ and IL-4 to determine the phenotypes of the immune responses. The data are presented as the mean ± S.D. Each sample was examined in triplicate. Results are representative of three independent experiments. *** (P < 0.001) indicates a statistically significant difference between the indicated groups as determined by one-way ANOVA. All treatment groups show significant differences when compared to the negative control groups (PBS and pcDNA3) (P < 0.05)
The co-administration of resiquimod and poly(I:C) with E7 DNA vaccine drives the development of intratumoural immunostimulatory cytokines in a tumor microenvironment
Activation of antigen-specific CD8+ T cells within spleens is essential for broad tumor immunity, as the tumor suppressive microenvironment may hinder T cell infiltration into the tumor site or interfere with their anti-tumor activity. Therefore, in order to assess the efficacy of the adjuvant co-administration, the concentrations of immunostimulatory and immuno-suppressive cytokines in the tumor microenvironment were determined. We investigated whether co-administration of resiquimod and poly(I:C) with E7 DNA vaccine could induce cytokine production in the tumor microenvironment. Mice with established TC-1 tumors were therefore co-administrated S.C. with toll-like receptor agonists and E7 DNA vaccine, and the tumor was excised 24 h after the last injection. After the extraction, the concentration of IL-12 and IL-10 were measured in the supernatant.
As shown in Fig. 4, mice immunized with the DNA vaccine produced significantly more IL-12 but less IL-10 than PBS- and pcDNA3- immunized mice (P < 0.05). While IL-12 (indicative of immunostimulatory responses) was increased when resiquimod and poly(I:C) were co-administered with E7 DNA, IL-10 (indicative of immunosuppressive responses) was decreased after the immunizations. Tumor lysate from the poly(I:C)-E7 DNA and resiquimod-E7 DNA groups produced the largest amounts of IL-12. Furthermore, the combination of poly(I:C)-E7 DNA and resiquimod-E7 DNA vaccine stimulated IL-12 production more than E7 DNA vaccine alone (P < 0.05). Tumor lysate from the poly(I:C)-E7 DNA and resiquimod- E7 DNA groups produced the smallest amounts of IL-10. Furthermore, the combination of poly(I:C)-E7 DNA and resiquimod-E7 DNA vaccine dampened IL-10 production more than E7 DNA vaccine alone (P < 0.01). These results demonstrate that TLR agonists in combination with E7 DNA vaccine prevent a strongly immunosuppressive milieu, which may be responsible for upregulating antitumor responses and tumor regression.
Assessment of the levels of pro-inflammatory and immunosuppressive cytokines in a tumor microenvironment. C57Bl/6 mice were challenged with 2 × 105 TC-1 tumor cells and vaccinated as described in the text. Twenty-four hours after the last injection, animals were sacrificed and homogenized to form a uniform cell suspension. The levels of IL-12 and IL-10 in tumor supernatants were analyzed by ELISA. Results are representative of three independent experiments with three mice per group ± S.D. *** (P < 0.001) and ** (P < 0.01) indicate statistically significant difference between the indicated groups as determined by one-way ANOVA. All treatment groups show significant differences when compared to the negative control groups (PBS and pcDNA3) (P < 0.05)
Therapeutic co-administration of resiquimod and poly(I:C) with E7 DNA cures mice with established E7-expressing tumors
To determine whether the observed increase in E7-specific immunity could be translated into a better E7-specific antitumor effect, we performed an in vivo tumor treatment experiment via a previously characterized E7-expressing tumor model, TC-1.
We investigated whether co-administration of resiquimod and poly(I:C) with E7 DNA vaccine could lead to regression of preexisting tumors. For this purpose, 2 × 105 TC-1 cells were first injected S.C. into C57BL/6 mice in the right flank. One week later, each mouse was treated with E7 DNA, poly(I:C)-E7 DNA, resiquimod-E7 DNA, control plasmid DNA or PBS three times at 7-day intervals. The tumors were measured twice a week once they became palpable. The tumor volume was monitored for 6 weeks after the tumor challenge.
As shown in Fig. 5, in mice receiving resiquimod-E7 DNA and poly(I:C)-E7 DNA vaccinations, the TC-1 tumors were eliminated from 90 % and 80 % of mice, respectively, whereas all of the mice receiving pcDNA3 plasmid or PBS developed tumors within 10 days after the inoculation of TC-1 cells. Thus, the tumor volume was significantly lower than in those in the pcDNA3 plasmid or PBS group (p = 0.02).
In vivo antitumor effects generated by treatment with poly(I:C)-E7 DNA, resiquimod-E7 DNA and E7 DNA alone. C57BL/6 mice were inoculated subcutaneously with 2 × 105 TC-1 tumor cells. Mice were then treated with combinations of TLR agonists and DNA vaccine as described in “Materials and methods”. Mice were monitored for tumor growth by measuring tumor diameters with calipers twice a week. Line and scatter plot graphs depicting the tumor volume (in mm3) are presented. The data presented represent three independent experiments
For the mice receiving E7 DNA vaccination, 40 % of those remained tumor-free 42 days after the TC-1 challenge. Additionally, the tumor volume in the resiquimod-E7 DNA and poly(I:C)-E7 DNA groups were significantly lower than that in the E7 DNA alone group (P < 0.001). As a control group, we also evaluated the antitumor effect of pcDNA3 + resiquimod or poly(I:C). The statistical analysis of tumor volume did not reveal significant differences between these groups and the pcDNA3-alone group (data not shown).
In summary, these results showed that co-administration of resiquimod and poly(I:C) with E7 DNA could significantly reduce tumor volume and eradicate the established E7-expressing tumors, whereas vaccination with E7 DNA alone failed to reduce established E7-expressing tumors in mice. This indicated that resiquimod and poly(I:C) adjuvants significantly promoted the antitumor immunity of the DNA vaccine.
Discussion
The Toll-like receptor (TLR) superfamily plays a principal role in the recognition of tumors and inducing immunity. Thirteen human TLRs have been identified to date. Each recognizes a distinct pathogen-associated molecular pattern (PAMP), and upon specific ligand binding, they trigger an antimicrobial innate immunity. Besides its involvement in the innate immune response, TLR engagement by specific ligands also shapes adaptive immunity [27].
Toll-like receptor agonists have been evaluated in experimental models as adjuvants for cancer vaccines. The basis of the therapy is that TLR agonists induce cell-mediated immune responses, in particular Th1 cells, NK cells and CD8 CTLs, which have been shown to promote anti-tumor immune responses, but it has not been demonstrated that the adjuvants eradicate tumor growth, although recent reports of studies using mouse models suggest that they may have some efficacy [3, 7].
To evaluate the antitumor activity of TLR agonists as adjuvant in combination with a DNA vaccine, tumor experiments were carried out to explore the potential combinatorial use of TLR ligands in vaccine development. In our study, mice were immunized subcutaneously with a DNA vaccine encoding HPV-16 E7 together with TLR3 and TLR8 ligands. A previous study has demonstrated that administering imiquimod and poly(I:C) in 20-μg doses was not optimal for tumor clearance; therefore, we administered the adjuvants at a dose of 50 μg per subcutaneous injection [15]. Our results showed that the combination of poly(I:C) (a TLR3 ligand) and resiquimod (a TLR7/8 ligand) with DNA vaccine synergistically induced significant CD8+ cytolytic activity, CD4+ T cell proliferation and interferon gamma secretion from pulsed lymphocytes that were specific for the HPV-16 E7 antigen. In contrast, there was little induction of CTL activity by delivery of DNA vaccine encoding E7.
This is supported by previous findings that antigen-specific CTL activity is induced by poly(I:C) [36, 37] and resiquimod [26, 38] in nonhuman primates, supporting that poly(I:C) and resiquimod co-delivery could be useful for induction of antigen-specific CTL activity and cellular immunity. Involvement of poly(I:C) [8] and resiquimod [5] in the production of IFN-gamma has been reported previously.
The responses indicated that resiquimod and poly(I:C) as DNA vaccine adjuvants were able to induce cellular immune response, which is critical in tumor eradication [18, 22]. Cytokine secretion analysis revealed that the cellular immune response could activate CTL to remove tumor cells with E7 antigen [13].
Administration of these two ligands as adjuvant in the E7-expressing tumor model TC-1 resulted in a reduction in tumor growth. Furthermore, the tumor experiments showed that combinational therapy led to tumor eradication in a majority of the C57BL/6 mice. However, they were not able to achieve complete tumor eradication in all animals subjected to the treatment. Resiquimod-E7 DNA and poly(I:C)-E7 DNA applications led to the regression of the TC-1 tumor from 90 % and 80 % of mice, respectively, while only 40 % regression was achieved in mice receiving DNA vaccination alone. This is compatible with previous findings of other groups. Dumitru et al. have demonstrated that TLR7/8 activation by resiquimod can induce antitumor effects through activation of antigen-presenting cell (APC) function, infiltration of effector T lymphocytes and NK cells with cytotoxic activity, and direct tumor-cell-specific apoptosis [9]. In agreement with our antitumor findings, it has been reported that poly(I:C)-induced apoptosis of cancer is mediated through TLR3 expressed by tumor cells [6, 31]. In other study for evaluation of poly(A:U) dsRNA, a therapeutic effect was mediated through TLR3 expressed on tumor cells, and this therefore represents an effective targeted treatment in patients with TLR3-positive cancers [33].
Intratumoral cytokine assays demonstrated that the administration of TLR agonists with the DNA vaccine results in substantial growth inhibition of pre-established tumors in C57BL/6 mice.
Our results showed that the TLR-agonist-mediated anti-tumor effect was associated with a significantly reduced secretion of immunosuppressive mediators and cytokines such as IL-10 and activation of the local immune regulators such as Il-12.
In previous studies, it has been shown that poly(I:C) stimulated monocyte-derived DCs to produce IL-12 and decrease their production of IL-10 [2]. TLR8-specific activation also induced IL-12 production by myeloid dendritic cells (mDCs) [19]. These activated DCs promoted antigen-specific CTL responses and the differentiation of CD4+ T cells toward a Th1 phenotype [25]. The findings are in accordance with our results showing that using TLR3 and 7 agonists as adjuvants skews the local intratumoral immunity toward Th1 polarization.
A comparison of the adjuvants used in this study showed that, in conjunction with resiquimod, the agonist even more effectively induced antigen-specific cytotoxic CD8+ T cells and enhanced expression of Th2-associated cytokines such as IL-4 against tumor challenge. It seems that there still might be subtle differences in the adjuvant effects between TLR3 and TLR7/8 agonists, although both eventually lead to similar levels of T cell activation. The TLR7/8 agonist is more effective in inducing both cellular immunity and regression of the tumor. Such divergence may imply variable mechanisms in response to different TLR ligands that may induce some different pathways [28].
For analysis of tumor control mechanisms, our results demonstrated that the subsequent administration of a TLR agonist with DNA vaccine will not only re-activate this immune system in the periphery, in a nonspecific manner against E7 antigen, but also mediate secretion of IL-12 as a pro-inflammatory cytokine by tumor cells, resulting in recruitment and intratumoral activation of effector cells. The conjoint TLR agonist administration will occur in a more efficient situation when a vaccine is first administered, as the vaccine will induce tumor-specific immunity, which will then be further re-stimulated and attracted to the tumor microenvironment by TLR3 and TLR7/8 agonists. Furthermore, after TLR treatment, tumor cells might also become more recognizable and therefore more susceptible to destruction by the immune system and display more TLR signals and thus promote pro-apoptotic activity. Therefore, the adjuvant activity of TLR agonists is due to an immunostimulating effect, reversal of the immunosuppressive network in the tumor microenvironment, and direct cytotoxic effect on tumor cells.
In conclusion, our study provides an outline for eradicating HPV-induced cancers involving a combination of poly(I:C) and resiquimod with an HPV DNA vaccine, followed by the induction of peripheral and circulating antigen-specific adaptive immunity and nonspecific intratumoral innate immunity to promote antitumor immunocytotoxic response to eradicate the lesions.
Abbreviations
- HPV:
-
Human papilloma virus
- TLR:
-
Toll-like receptor
- PHA:
-
Phytohemagglutinin
- APC:
-
Antigen-presenting cell
- CTL:
-
Cytolytic T lymphocyte
- DC:
-
Dendritic cell
- FDA:
-
Food and Drug Administration
- IFN- γ:
-
Interferon γ
- IL-4:
-
Interleukin 4
- IL-10:
-
Interleukin 10
- IL-12:
-
Interleukin 12
- PMSF:
-
Phenylmethanesulfonyl fluoride
- LDH:
-
Lactate dehydrogenase
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OD:
-
Optical density
- FCS:
-
Fetal calf serum
- RPMI:
-
1640 Roswell Park Memorial Institute (name of the medium)
- Th:
-
T helper
References
WHO/ICO HPV Information Centre (2010) Human papillomavirus and related cancers in world. Summary Report 2010 WHO
Adams M, Navabi H, Jasani B, Man S, Fiander A, Evans AS, Donninger C, Mason M (2003) Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R). Vaccine 21:787–790
Adams S (2009) Toll-like receptor agonists in cancer therapy. Immunotherapy 1:949–964
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738
Brugnolo F, Sampognaro S, Liotta F, Cosmi L, Annunziato F, Manuelli C, Campi P, Maggi E, Romagnani S, Parronchi P (2003) The novel synthetic immune response modifier R-848 (Resiquimod) shifts human allergen-specific CD4+ TH2 lymphocytes into IFN-gamma-producing cells. J Allergy Clin Immunol 111:380–388
Cheng YS, Xu F (2010) Anticancer function of polyinosinic-polycytidylic acid. Cancer Biol Ther 10:1219–1223
Chuang CM, Monie A, Hung CF, Wu TC (2010) Treatment with imiquimod enhances antitumor immunity induced by therapeutic HPV DNA vaccination. J Biomed Sci 17:32
Dias CC, Moraes MP, Weiss M, Diaz-San Segundo F, Perez-Martin E, Salazar AM, de los Santos T, Grubman MJ (2012) Novel antiviral therapeutics to control foot-and-mouth disease. J Interferon Cytokine Res 32:462–473
Dumitru CD, Antonysamy MA, Gorski KS, Johnson DD, Reddy LG, Lutterman JL, Piri MM, Proksch J, McGurran SM, Egging EA, Cochran FR, Lipson KE, Tomai MA, Gullikson GW (2009) NK1.1+ cells mediate the antitumor effects of a dual Toll-like receptor 7/8 agonist in the disseminated B16-F10 melanoma model. Cancer Immunol Immunother 58:575–587
Fidock MD, Souberbielle BE, Laxton C, Rawal J, Delpuech-Adams O, Corey TP, Colman P, Kumar V, Cheng JB, Wright K, Srinivasan S, Rana K, Craig C, Horscroft N, Perros M, Westby M, Webster R, van der Ryst E (2011) The innate immune response, clinical outcomes, and ex vivo HCV antiviral efficacy of a TLR7 agonist (PF-4878691). Clin Pharmacol Ther 89:821–829
Fife KH, Meng TC, Ferris DG, Liu P (2008) Effect of resiquimod 0.01% gel on lesion healing and viral shedding when applied to genital herpes lesions. Antimicrob Agents Chemother 52:477–482
Ghaemi A, Soleimanjahi H, Gill P, Hassan Z, Jahromi SR, Roohvand F (2010) Recombinant lambda-phage nanobioparticles for tumor therapy in mice models. Genet Vaccines Ther 8:3
Ghaemi A, Soleimanjahi H, Gill P, Hassan ZM, Razeghi S, Fazeli M, Razavinikoo SM (2011) Protection of mice by a lambda-based therapeutic vaccine against cancer associated with human papillomavirus type 16. Intervirology 54:105–112
Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP (2002) Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol 218:74–86
Gnjatic S, Sawhney NB, Bhardwaj N (2010) Toll-like receptor agonists: are they good adjuvants? Cancer J (Sudbury, Mass) 16:382–391
Guy B (2007) The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol 5:505–517
Huang B, Mao CP, Peng S, He L, Hung CF, Wu TC (2007) Intradermal administration of DNA vaccines combining a strategy to bypass antigen processing with a strategy to prolong dendritic cell survival enhances DNA vaccine potency. Vaccine 25:7824–7831
Huang CY, Chen JJ, Shen KY, Chang LS, Yeh YC, Chen IH, Chong P, Liu SJ, Leng CH (2012) Recombinant lipidated HPV E7 induces a Th-1-biased immune response and protective immunity against cervical cancer in a mouse model. PloS One 7:e40970
Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S (2002) Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med 195:1507–1512
Lin K, Roosinovich E, Ma B, Hung CF, Wu TC (2010) Therapeutic HPV DNA vaccines. Immunol Res 47:86–112
Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM (2009) Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med 206:1589–1602
Marquez JP, Rivera R, Kang KH, Gardner MB, Torres JV (2012) Human papillomavirus immunogen that provides protective tumor immunity and induces tumor regression. Viral Immunol 25:141–152
McLaughlin-Drubin ME, Munger K (2009) The human papillomavirus E7 oncoprotein. Virology 384:335–344
Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, Shah KV, Meijer CJ (2004) Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer 111:278–285
Navabi H, Jasani B, Reece A, Clayton A, Tabi Z, Donninger C, Mason M, Adams M (2009) A clinical grade poly I:C-analogue (Ampligen) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine 27:107–115
Otero M, Calarota SA, Felber B, Laddy D, Pavlakis G, Boyer JD, Weiner DB (2004) Resiquimod is a modest adjuvant for HIV-1 gag-based genetic immunization in a mouse model. Vaccine 22:1782–1790
Pasare C, Medzhitov R (2004) Toll-like receptors: linking innate and adaptive immunity. Microbes Infect 6:1382–1387
Pulendran B (2005) Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol (Baltimore, Md : 1950) 174:2457–2465
Qin H, Cha SC, Neelapu SS, Lou Y, Wei J, Liu YJ, Kwak LW (2009) Vaccine site inflammation potentiates idiotype DNA vaccine-induced therapeutic T cell-, and not B cell-, dependent antilymphoma immunity. Blood 114:4142–4149
Saade F, Petrovsky N (2012) Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines 11:189–209
Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T (2006) TLR3 can directly trigger apoptosis in human cancer cells. J Immunol (Baltimore, Md : 1950) 176:4894–4901
Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P (2007) Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin Cancer Res 13:4565–4574
Salaun B, Zitvogel L, Asselin-Paturel C, Morel Y, Chemin K, Dubois C, Massacrier C, Conforti R, Chenard MP, Sabourin JC, Goubar A, Lebecque S, Pierres M, Rimoldi D, Romero P, Andre F (2011) TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res 71:1607–1614
Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C (2005) Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887–892
Su JH, Wu A, Scotney E, Ma B, Monie A, Hung CF, Wu TC (2010) Immunotherapy for cervical cancer: research status and clinical potential. BioDrugs 24:109–129
Tewari K, Flynn BJ, Boscardin SB, Kastenmueller K, Salazar AM, Anderson CA, Soundarapandian V, Ahumada A, Keler T, Hoffman SL, Nussenzweig MC, Steinman RM, Seder RA (2010) Poly(I:C) is an effective adjuvant for antibody and multi-functional CD4+ T cell responses to Plasmodium falciparum circumsporozoite protein (CSP) and alphaDEC-CSP in non human primates. Vaccine 28:7256–7266
Trumpfheller C, Longhi MP, Caskey M, Idoyaga J, Bozzacco L, Keler T, Schlesinger SJ, Steinman RM (2012) Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med 271:183–192
Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA (2005) Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol (Baltimore, Md: 1950) 174:7676–7683
Yu L, Chen S (2008) Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunol Immunother 57:1271–1278
Funding
This study was supported by Golestan University of Medical Sciences, Gorgan, Iran.
Disclosure
All the authors declare that they have no conflicting interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajadian, A., Tabarraei, A., Soleimanjahi, H. et al. Comparing the effect of Toll-like receptor agonist adjuvants on the efficiency of a DNA vaccine. Arch Virol 159, 1951–1960 (2014). https://doi.org/10.1007/s00705-014-2024-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-014-2024-4