Abstract
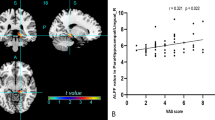
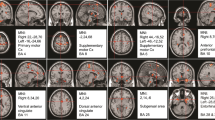
In patients with Parkinson’s disease (PD), abnormal activations of nociceptive brain areas and lowered pain thresholds were reported, probably reflecting a central modification of pain processing. The aim of this study was to investigate the possible correlation between the striatal and extrastriatal dopaminergic system and pain threshold in PD patients. We included 25 PD patients with various intensities of central pain (visual analog scale). Subjective pain threshold (thermotest) and a motor examination (UPDRS III) were performed. Patients underwent SPECT imaging with [123I]-FP-CIT. We analyzed the correlation between [123I]-FP-CIT binding and subjective pain threshold, using a simple linear regression model for striatal uptake and a voxel-based approach for extrastriatal uptake. The covariables were age, sex, duration of PD, and UPDRS motor score. A pain matrix mask was also used to identify clusters in relation with pain matrix. Striatal analysis revealed that [123I]-FP-CIT binding was negatively correlated with age (p = 0.02), duration of PD (p = 0.0002) and UPDRS motor score (p = 0.006), but no correlation with pain threshold was observed. The extrastriatal analysis showed a positive correlation between [123I]-FP-CIT binding and subjective heat pain threshold for the left posterior cingulate cortex (PCC) (p < 0.001) and negative correlations for the right secondary visual cortex (p < 0.001) and left insula (p < 0.001). When applying the pain matrix mask, correlations remained significant only in the left PCC and the left insula. We suggest that pain perception abnormalities in PD are not directly related to striatal dopaminergic dysfunction. Painful sensations may be related to extrastriatal monoaminergic systems.


Similar content being viewed by others
References
Becerra LR et al (1999) Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med 41:1044–1057
Beiske AG, Loge JH, Ronningen A, Svensson E (2009) Pain in Parkinson’s disease prevalence characteristics. Pain 141:173–177
Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG (2000) Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 15:692–698
Brefel-Courbon C et al (2005) Effect of levodopa on pain threshold in Parkinson’s disease: a clinical and positron emission tomography study. Mov Disord 20:1557–1563
Brefel-Courbon C et al (2009) Comparison of chronic analgesic drugs prevalence in Parkinson’s disease other chronic diseases the general population. Pain 141:14–18
Brefel-Courbon C, Ory-Magne F, Thalamas C, Payoux P, Rascol O (2013) Nociceptive brain activation in patients with neuropathic pain related to Parkinson’s disease. Parkinsonism Relat Disord 19:548–552
Bromm B (2001) Brain images of pain. News Physiol Sci 16:244–249
Chudler EH, Dong WK (1995) The role of the basal ganglia in nociception and pain. Pain 60:3–38
Coffeen U, Ortega-Legaspi JM, de Gortari P, Simon-Arceo K, Jaimes O, Amaya MI, Pellicer F (2010) Inflammatory nociception diminishes dopamine release and increases dopamine D2 receptor mRNA in the rat’s insular cortex. Mol Pain 6:75
Coghill RC, Sang CN, Maisog JM, Iadarola MJ (1999) Pain intensity processing within the human brain: a bilateral distributed mechanism. J Neurophysiol 82:1934–1943
Defazio G et al (2008) Pain as a nonmotor symptom of Parkinson disease: evidence from a case–control study. Arch Neurol 65:1191–1194. https://doi.org/10.1001/archneurol.2008.2
Defrin R, Pick CG, Peretz C, Carmeli E (2004) A quantitative somatosensory testing of pain threshold in individuals with mental retardation. Pain 108:58–66
Dellapina E et al (2012) Effect of subthalamic deep brain stimulation on pain in Parkinson‘s disease. Pain 153:2267–2273
Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D (2004) Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 62:2171–2175
Fruhstorfer H, Lindblom U, Schmidt WC (1976) Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry 39:1071–1075
Garcia-Larrea L (2012) The posterior insular-opercular region and the search of a primary cortex for pain. Neurophysiol Clin 42:299–313
Gerdelat-Mas A, Simonetta-Moreau M, Thalamas C, Ory-Magne F, Slaoui T, Rascol O, Brefel-Courbon C (2007) Levodopa raises objective pain threshold in Parkinson’s disease: a RIII reflex study. J Neurol Neurosurg Psychiatry 78:1140–1142
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M (1995) Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain 63:225–236
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Hurd YL, Suzuki M, Sedvall GC (2001) D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat 22:127–137
Kas A et al (2007) Validation of a standardized normalization template for statistical parametric mapping analysis of 123I-FP-CIT images. J Nucl Med 48:1459–1467
Kumru H, Soler D, Vidal J, Tormos JM, Pascual-Leone A, Valls-Sole J (2012) Evoked potentials and quantitative thermal testing in spinal cord injury patients with chronic neuropathic pain. Clin Neurophysiol 123:598–604
Lim SY, Farrell MJ, Gibson SJ, Helme RD, Lang AE, Evans AH (2008) Do dyskinesia and pain share common pathophysiological mechanisms in Parkinson’s disease? Mov Disord 23:1689–1695
Moisset X, Bouhassira D (2007) Brain imaging of neuropathic pain. Neuroimage 37(Suppl 1):S80–S88
Mylius V et al (2009) Pain sensitivity and descending inhibition of pain in Parkinson’s disease. J Neurol Neurosurg Psychiatry 80:24–28
Negre-Pages L, Regragui W, Bouhassira D, Grandjean H, Rascol O (2008) Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord 23:1361–1369
Nielsen FA, Balslev D, Hansen LK (2005) Mining the posterior cingulate: segregation between memory pain components. Neuroimage 27:520–532
Peyron R, Laurent B, Garcia-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30:263–288
Schestatsky P, Kumru H, Valls-Sole J, Valldeoriola F, Marti MJ, Tolosa E, Chaves ML (2007) Neurophysiologic study of central pain in patients with Parkinson disease. Neurology 69:2162–2169
Slaoui T, Mas-Gerdelat A, Ory-Magne F, Rascol O, Brefel-Courbon C (2007) [Levodopa modifies pain thresholds in Parkinson’s disease patients]. Rev Neurol (Paris) 163:66–71 (MDOI-RN-01-2007-163-1-0035-3787-101019-200604714 [pii])
Stern AF (2014) The hospital anxiety and depression scale. Occup Med 64:393–394 https://doi.org/10.1093/occmed/kqu024
Tinazzi M et al (2008) Abnormal processing of the nociceptive input in Parkinson’s disease: a study with CO2 laser evoked potentials. Pain 136:117–124
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS (2006) Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging 33:1491–1499. https://doi.org/10.1007/s00259-006-0155-x
Volkow ND et al (1996) Dopamine transporters decrease with age. J Nucl Med 37:554–559
Wasner G, Deuschl G (2012) Pains in Parkinson disease–many syndromes under one umbrella. Nat Rev Neurol 8:284–294
Xie YF, Huo FQ, Tang JS (2009) Cerebral cortex modulation of pain. Acta Pharmacol Sin 30:31–41
Zambito Marsala S et al (2010) Spontaneous pain, pain threshold, and pain tolerance in Parkinson’s disease. J Neurol 258:627–633
Acknowledgements
This work was supported by a Toulouse University Hospital grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dellapina, E., Pellaprat, J., Adel, D. et al. Dopaminergic denervation using [123I]-FPCIT and pain in Parkinson’s disease: a correlation study. J Neural Transm 126, 279–287 (2019). https://doi.org/10.1007/s00702-019-01974-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-01974-5